|
CARBON DIOXIDE
|
|
Carbon dioxide is a chemical compound composed of one carbon and two oxygen atoms. It is often referred to by its formula CO2. It is present in the Earth's atmosphere at a low concentration and acts as a greenhouse gas. In its solid state, it is called dry ice. It is a major component of the carbon cycle.
Origins of CO2
Atmospheric carbon dioxide derives from multiple natural sources including volcanic outgassing, the combustion of organic matter, and the respiration processes of living aerobic organisms; man-made sources of carbon dioxide come mainly from the burning of various fossil fuels for power generation and transport use. It is also produced by various microorganisms from fermentation and cellular respiration. Plants convert carbon dioxide to oxygen during a process called photosynthesis, using both the carbon and the oxygen to construct carbohydrates. In addition, plants also release oxygen to the atmosphere, which is subsequently used for respiration by heterotrophic organisms, forming a cycle.
Smoke and ozone pollution from Indonesian fires 1997
Chemical and physical properties
Carbon dioxide is a colorless gas which, when inhaled at high concentrations (a dangerous activity because of the associated asphyxiation risk), produces a sour taste in the mouth and a stinging sensation in the nose and throat. These effects result from the gas dissolving in the mucous membranes and saliva, forming a weak solution of carbonic acid. You may notice this sensation if you attempt to stifle a burp after drinking a carbonated beverage.
Its density at 25 °C is 1.98 kg m-3, about 1.65 times that of air. The carbon dioxide molecule (O=C=O) contains two double bonds and has a linear shape. It has no electrical dipole. As it is fully oxidized, it is not very reactive and, in particular, not flammable.
At temperatures below −78 °C, carbon dioxide changes directly from a gas to a white solid called dry ice through a process called deposition. Liquid carbon dioxide forms only at pressures above 5.1 atm; at atmospheric pressure, it passes directly between the solid phase and the gaseous phase in a process called sublimation.
Carbon dioxide is soluble in water, in which it spontaneously interconverts between CO2 and H2CO3 (carbonic acid). The relative concentrations of CO2, H2CO3, and the deprotonated forms HCO3- (bicarbonate) and CO32-(carbonate) depend on pH. In neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater, while in very alkaline water (pH > 10.4) the predominant (>50%) form is carbonate. The bicarbonate and carbonate forms are very soluble, such that air-equilibrated ocean water (mildly alkaline with typical pH = 8.2-8.5) contains about 120 mg of bicarbonate per liter.
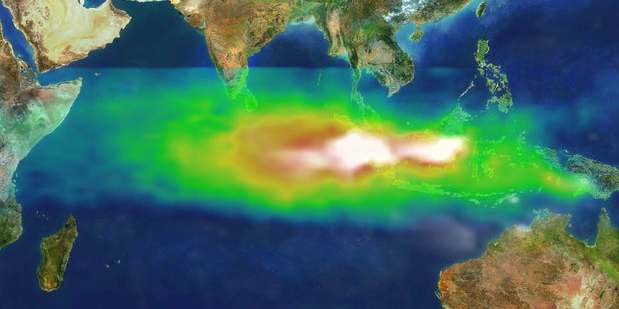
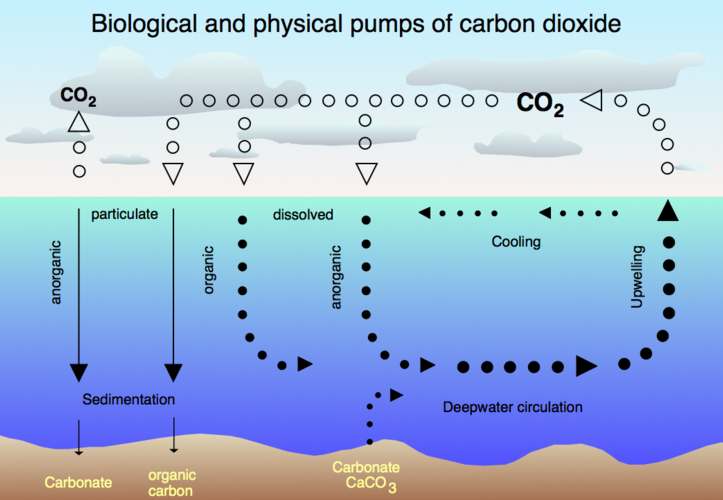
Air-sea exchange of CO2
Oceans
The Earth's oceans contain a huge amount of carbon dioxide in the form of bicarbonate and carbonate ions—much more than the amount in the atmosphere. The bicarbonate is produced in reactions between rock, water, and carbon dioxide. One example is the dissolution of calcium carbonate:
Reactions like this tend to buffer changes in atmospheric CO2. Reactions between carbon dioxide and non-carbonate rocks also add bicarbonate to the seas, which can later undergo the reverse of the above reaction to form carbonate rocks, releasing half of the bicarbonate as CO2. Over hundreds of millions of years this has produced huge quantities of carbonate rocks. If all the carbonate rocks in the earth's crust were to be converted back into carbon dioxide, the resulting carbon dioxide would weigh 40 times as much as the rest of the atmosphere.
The vast majority of CO2 added to the atmosphere will eventually be absorbed by the oceans and become bicarbonate ion, but the process takes on the order of a hundred years because most seawater rarely comes near the surface.
History
Carbon dioxide was one of the first gases to be described as a substance distinct from air. In the seventeenth century, the Flemish chemist Jan Baptist van Helmont observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a "gas" or "wild spirit" (spiritus sylvestre).
Carbon dioxide's properties were studied more thoroughly in the 1750s by the Scottish physician Joseph Black. He found that limestone (calcium carbonate) could be heated or treated with acids to yield a gas he termed "fixed air." He observed that the fixed air was denser than air and did not support either flame or animal life. He also found that it would, when bubbled through an aqueous solution of lime (calcium hydroxide), precipitate calcium carbonate, and used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, Joseph Priestley used carbon dioxide produced from the action of sulfuric acid on limestone to prepare soda water, the first known instance of an artificially carbonated drink.
Carbon dioxide was first liquefied (at elevated pressures) in 1823 by Humphry Davy and Michael Faraday. The earliest description of solid carbon dioxide was given by Charles Thilorier, who in 1834 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a "snow" of solid CO2.
LINKS and REFERENCE
A taste for adventure capitalists
Solar Cola - a healthier alternative
|
|
This website is Copyright © 1999 & 2018. The names Elizabeth Swan and Kulo Luna are trademarks. All rights reserved. All other trademarks are hereby acknowledged. Max Energy Limited is an educational charity. |