|
GLASS FIBER REINFORCED PLASTIC
|
||||||||||
|
Composite materials (or composites for short) are engineered materials made from two or more constituent materials that remain separate and distinct on a macroscopic level while forming a single component.
There are two categories of constituent materials: matrix and reinforcement. At least one portion (fraction) of each type is required. The matrix material surrounds and supports the reinforcement materials by maintaining their relative positions. The reinforcements impart special physical (mechanical and electrical) properties to enhance the matrix properties. A synergism produces material properties unavailable from naturally occurring materials. Due to the wide variety of matrix and reinforcement materials available, the design potential is incredible.
Glassfiber roving strands
This great variety has resulted in an enormous lexicon that confounds both new and experienced students. Names and descriptors arise from the respective experiences of different perspectives. While different industries use different terms to describe the same things, the same term can be applied in vastly different contexts.
The most primitive composite materials comprised straw and mud in the form of bricks for building construction. The most advanced examples perform routinely on spacecraft in demanding environments. The most visible applications pave our roadways in the form of either steel and aggregate reinforced portland cement or asphalt concrete. Those composites closest to our personal hygiene form our shower stalls and bath tubs made of fiberglass. Solid surface, imitation granite and cultured marble sinks and countertops are widely used.
There are the so-called natural composites like bone and wood. Both of these are constructed by the processes of nature and beyond the scope of this text. Engineered composite materials must be formed to shape. This involves strategically placing the reinforcements while manipulating the matrix properties to achieve a melding event at or near the beginning of the component life cycle. A variety of methods are used according to the end item design requirements. These fabrication methods are commonly named molding or casting processes, as appropriate, and both have numerous variations.
The principle factors impacting the methodology are the natures of the chosen matrix and reinforcement materials. Another important factor is the gross quantity of material to be produced. Large quantities can be used to justify high capital expenditures for rapid and automated manufacturing technology. Small production quantities are accommodated with lower capital expenditures but higher labor costs at a correspondingly slower rate.
Many commercially produced composites use a polymer matrix material often called a resin or resin solution. There are many different polymers available depending upon the starting raw ingredients. There are several broad categories, each with numerous variations. The most common categories are known as polyester, vinyl ester, epoxy, phenolic, polyimide, polyamide, and others. The reinforcement materials are often fibers but also commonly ground minerals. Fibers are often transformed into a textile material such as a felt, fabric, knit or stitched construction.
Rutan composite aircraft
Advanced composite materials constitute a category comprising carbon fiber reinforcement and epoxy or polyimide matrix materials. These are the aerospace grade composites and typically involve laminate molding at high temperature and pressure to achieve high reinforcement volume fractions. These advanced composite materials feature high stiffness and/or strength to weight ratios.
One component is often a strong fibre such as fiberglass, quartz, kevlar, Dyneema or carbon fibre that gives the material its tensile strength, while another component (called a matrix) is often a resin such as polyester, or epoxy that binds the fibres together, transferring load from broken fibers to unbroken ones and between fibers that are not oriented along lines of tension. Also, unless the matrix chosen is especially flexible, it prevents the fibers from buckling in compression. Some composites use an aggregate instead of, or in addition to, fibers.
In terms of stress, any fibers serve to resist tension, the matrix serves to resist shear, and all materials present serve to resist compression, including any aggregate. Composite materials can be divided into two main categories normally referred to as short fiber reinforced materials and continuous fiber reinforced materials. Continuous reinforced materials will often constitute a layered or laminated structure.
CARBON FIBER
Carbon fiber can refer to carbon filament thread, or to felt or woven cloth made from those carbon filaments. By extension, it is also used informally to mean any composite material made with carbon filament. It is a strong and very expensive material.
Synthesis
Each carbon filament is made out of long, thin sheets of carbon similar to graphite. A common method of making carbon filaments is the oxidation and thermal pyrolysis of polyacrylonitrile (PAN), a polymer used in the creation of many synthetic materials. Like all polymers, polyacrylonitrile molecules are long chains, which are aligned in the process of drawing fibres. When heated in the correct fashion, these chains bond side-to-side, forming narrow graphene sheets which eventually merge to form a single, jelly roll-shaped filament. The result is usually 93-95% carbon. Lower-quality fiber can be manufactured using pitch or rayon as the precursor instead of PAN. The carbon can become further enhanced, as high modulus, or high strength carbon, by heat treatment processes. Carbon heated in the range of 1500-2000°C (carburizing) exhibits the highest tensile strength (820,000 Psi or 5,650 N/mm²), while carbon fiber heated from 2500-3000°C (graphitizing) exhibits a higher modulus of elasticity (77,000,000 Psi or 531 kN/mm²).
Textile
These filaments are stranded into a thread. Carbon fiber thread is rated by the number of filaments per thread, in thousands. For example, 3K (3,000 filament) carbon fiber is 3 times as strong as 1K carbon fiber, but is also 3 times as heavy. This thread can then be used to weave a carbon fiber cloth. The appearance of this cloth generally depends on the size of thread and the weave chosen. Carbon fiber is naturally a glossy black but recently colored carbon fiber has become available.
Uses
Carbon fiber is most notably used to reinforce composite materials, particularly the class of materials known as graphite reinforced plastic. This class of materials is used in high-performance vehicles, sporting equipment, and other demanding mechanical applications; a more thorough discussion of these uses, including composite lay-up techniques, can be found in the carbon fiber composite article.
Non-polymer materials can also be used as the matrix for carbon fibres. Due to the formation of metal carbides (i.e., water-soluble AlC) and corrosion considerations, carbon has seen limited success in metal matrix composite applications. Reinforced carbon-carbon (RCC) consists of carbon fibre-reinforced graphite, and is used structurally in high-temperature applications, such as the nose cone and leading edges of the space shuttle.
The fibre also finds use in filtration of high-temperature gases, as an electrode with high surface area and impeccable corrosion resistance, and as an anti-static component in high-performance clothing.
Some string instruments, such as violins and cellos, use carbon fibre reinforced composite bows. This is an alternative to the more common wooden bows.
Many high end frames for road bikes and mountain bikes are made of carbon fiber reinforced composite. Also, many road bikes made of aluminum have carbon fiber reinforced composite seat posts, handlebars and forks for reduced weight.
Future Directions
Carbon nanotubes are currently being investigated as possible improvements on the traditional carbon fiber material. While the nanotechnology field isn't advanced enough to create long-enough fibers made entirely of carbon nanotubes, research has shown that even as little as 5% (by weight) carbon nanotube constituents within the carbon fibers will dramatically improve properties. Andrews et. al. reported [1] that tensile strength increased by 90%, modulus increased by 150%, and electrical conductivity increased by 340%. This was in a pitch composite fiber with 5% (by weight) loading of purified single walled nanotubes (as compared to the corresponding values in unmodified isotropic pitch fibers). Further research is still needed to resolve issues such as nanotube dispersion and alignment, as well as interfacial bonding; however, this approach holds great promise for improving both the mechanical and electrical properties of carbon fiber composites.
Bluebird BE2 GRP bodywork
Automotive uses
CFRP is used extensively in automobile racing, most especially in Formula One and Indycar racing. The high cost of carbon fiber is mitigated by the material's unsurpassed strength-to-weight ratio, and low weight is essential for high-performance automobile racing. Racecar manufacturers have also developed methods to give carbon fiber pieces strength in a certain direction, making it strong in a load-bearing direction, but weak in directions where little or no load would be placed on the member. Conversely, manufacturers developed omnidirectional carbon fiber weaves that apply strength in all directions. This type of carbon fiber assembly is most widely used in the "safety cell" monocoque chassis assembly of high-performance racecars.
In 2000, Ferrari's factory Formula One racing team, long at the forefront of development of carbon fiber technology for racing vehicles, discovered a loophole in the Formula One rule book. Ferrari developed a rear wing that would deflect downward at high speed, thus changing the angle of attack of the wing in order to reduce drag. Downforce was also affected, but compensated for with design of the car's underbody and rear diffuser. Several supercars over the past few decades have incorporated CFRP extensively in their manufacture, using it for their monocoque chassis as well as other components. Examples include the Koenigsegg ccR, McLaren F1, Bugatti Veyron, Bugatti EB110, Pagani Zonda, Ferrari Enzo and Porsche Carrera GT.
Until recently, the material has had limited use in mass-produced cars because of the expense involved in terms of materials, equipment, and the relatively limited pool of individuals with expertise in working with it. Recently, several mainstream vehicle manufacturers such as General Motors and BMW have started to use carbon fiber technology in everyday road cars.
Chevrolet is using carbon fiber in its flagship sports car, the Corvette. A special high performance version of the Corvette, dubbed the Z06, includes carbon fiber front bodywork for reduced weight and added rigidity.
BMW produces carbon fiber reinforced plastics in its Landshut plant. To make the roof of the BMW M3 CSL, for example, 5 layers of carbon fiber cloth are placed in an 1,800 ton press, where epoxy is resin transfer molded and heat-cured in a robot-automated process. The resulting roof is half the weight of an equivalent steel roof.
Use of the material has been more readily adopted by low-volume manufacturers like TVR who use it primarily for creating body-panels for some of their high-end cars due to its increased strength and decreased weight compared with the glass-reinforced plastic they use for the majority of their products.
KEVLAR
Kevlar is the DuPont Company's brand name for material made out of synthetic fiber of poly-paraphenylene terephthalamide which is constructed of para-aramid fibers that the company claims is five times stronger than the same weight of steel, while being lightweight, flexible and comfortable. It is also very heat resistant and decomposes above 400 °C without melting. It was invented by Stephanie Kwolek of DuPont from research into high performance polymers, and patented by her in 1966 and first marketed in 1971. Kevlar is a registered trademark of E.I. du Pont de Nemours and Company.
Originally intended to replace the steel belts in tires, it is probably the most well known name in soft armor as bulletproof vests. It is also used in extreme sports equipment, high-tension drumhead applications, animal handling protection, composite aircraft construction, fire suits, yacht sails, and as an asbestos replacement.
When this polymer is spun in the same way that a spider spins a web, the resulting commercial para-aramid fiber has tremendous strength, and is heat and cut resistant. Para-aramid fibers do not rust or corrode, and their strength is unaffected by immersion in water. When woven together, they form a good material for mooring lines and other underwater objects. However, unless specially waterproofed, para-aramid fiber’s ability to stop bullets and other projectiles is degraded when wet.
Properties
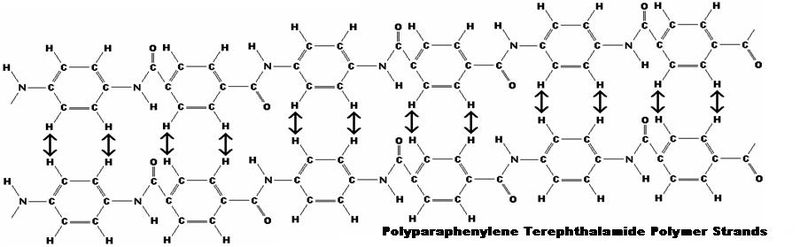
Kevlar is a type of aramid that consists of long polymeric chains with a parallel orientation. Kevlar derives its strength from inter-molecular hydrogen bonds and aromatic stacking interactions between aromatic groups in neighboring strands. These interactions are much stronger than the van der Waals interaction found in other synthetic polymers and fibers like Dyneema. The presence of salts and certain other impurities, especially calcium, would interfere with the strand interactions and has to be avoided in the production process. Kevlar consists of relatively rigid molecules,which form a planar sheet-like structure similar to silk protein.
Polyparaphenylene Terephthalamide Intermolecular Hydrogen Bonding
These properties result in its high mechanical strength and its remarkable heat resistance. Because it is highly unsaturated, as the ratio of carbon to hydrogen atoms is quite high, it has a low flammability. Kevlar molecules have polar groups accessible for hydrogen bonding. Water that enters the interior of the fiber can take the place of bonding between molecules and reduce the material's strength, while the available groups at the surface lead to good wetting properties. This is important for bonding the fibers to other types of polymer, forming a fibre reinforced plastic. This same property also makes the fibers feel more natural and "sticky" compared to nonpolar polymers like polyethylene.
In structural applications, Kevlar fibers can be bonded to one another or to other materials to form a composite. Kevlar's main weaknesses are that it decomposes under alkaline conditions or when exposed to chlorine. While it can have a great tensile strength, sometimes in excess of 4.0 GPa, like all fibers it tends to buckle in compression.
Production
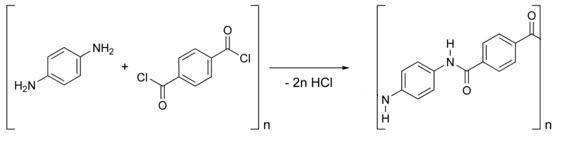
Kevlar is synthesized from the monomers 1,4-phenyl-diamine (para-phenylenediamine) and terephthaloyl chloride. The result is a polymeric aromatic amide (aramid) with alternating benzene rings and amide groups. When they are produced, these polymer strands are aligned randomly. To make Kevlar, they are dissolved and spun, causing the polymer chains to orientate in the direction of the fibre.
Kevlar has a high price, in part, due to the difficulties arising from the use of concentrated sulfuric acid in its manufacture. These harsh conditions are needed to keep the highly insoluble polymer in solution during synthesis and spinning.
Kevlar Synthesis
GRP GLASS REINFORCED PLASTIC
Glass-reinforced plastic (GRP), is a composite material or fibre reinforced plastic made of a plastic reinforced by fine fibers made of glass. Like graphite-reinforced plastic, the composite material is commonly referred to by the name of its reinforcing fibers (fiberglass), an example of part-for-whole metonymy. The plastic is most often polyester or vinylester, but other plastics, like epoxy (GRE), are also sometimes used. The glass is mostly in the form of chopped strand mat (CSM), but woven fabrics are also used.
GRP/GRE is a versatile material with many uses. Although GRP was originally developed in the UK during the Second World War as a replacement for the molded plywood used in aircraft radomes (GRP being transparent to microwaves) its first main civilian application was for building of boats, where it gained acceptance in the 1950s, and now plays a dominant role. But its use has broadened over the years, and it is used extensively within the automotive and sport equipment sectors, although its use there is being taken over by carbon fiber because of its lower weight. GRE is also used to make hot tubs, pipes for drinking water, sewers, chemicals, and so on.
Advanced manufacturing techniques such as pre-pregs and fibre rovings extend the applications and the tensile strength possible with fibre-reinforced plastics.
GRP is also widely used in the telecommunications industry for shrouding the visual appearance of antennas, due to its RF permeability and low signal attenuation properties. It may also be used to shroud the visual appearance of other equipment where no signal permeability is required, such as equipment cabinets and steel support structures, due to the ease with which it can be moulded, manufactured, and painted to custom designs, to blend in with existing structures or brickwork. Examples of GRP usage
Fiberglass or fibreglass is material made from extremely fine fibers of glass. It is used as a reinforcing agent for many plastic products; the resulting composite material, properly known as glass-reinforced plastic (GRP) or glass-fiber reinforced epoxy (GRE), is called "fiberglass" in popular usage.
Glassmakers throughout history have experimented with glass fibers, but mass manufacture of fiberglass was only made possible with the advent of finer machine-tooling. In 1893, Edward Drummond Libbey exhibited a dress at the World's Columbian Exposition incorporating glass fibers with the diameter and texture of silk fibers. What is commonly known as "fiberglass" today, however, was invented in 1938 by Russell Games Slayter of Owens-Corning as a material to be used as insulation. It is marketed under the trade name Fiberglas (sic), see also genericized trademark.
Formation
Glass fiber is formed when thin strands of silica-based or other formulation glass is extruded into many fibers with small diameters suitable for textile processing. Glass is unlike other polymers in that, even as a fiber, it has little crystalline structure (see amorphous solid). The properties of the structure of glass in its softened stage are very much like its properties when spun into fiber. One definition of glass is "an inorganic substance in a condition which is continuous with, and analogous to the liquid state of that substance, but which, as a result of a reversible change in viscosity during cooling, has attained so high a degree of viscosity as to be for all practical purposes rigid."
The technique of heating and drawing glass into fine fibers has been known to exist for thousands of years; however, the concept of using these fibers for textile applications is more recent. The first commercial production of fiberglass was in 1936. In 1938, Owens-Illinois Glass Company and Corning Glass Works joined to form the Owens-Corning Fiberglas Corporation. Until this time all fiberglass had been manufactured as staple. When the two companies joined together to produce and promote fiberglass, they introduced continuous filament glass fibers. Owens-Corning is still the major fiberglass producer in the market today.
Chemistry
The basis of textile grade glass fibers is silica, SiO2. In its pure form it exists as a polymer, (SiO2)n. It has no true melting point but softens up to 2000°C, where it starts to degrade. At 1713°C, most of the molecules can move about freely. If the glass is then cooled quickly, they will be unable to form an ordered structure. In the polymer it forms SiO4 groups which are configured as a tetrahedron with the silicon atom at the center, and four oxygen atoms at the corners. These atoms then form a network bonded at the corners by sharing the oxygen atoms.
The vitreous and crystalline states of silica (glass and quartz) have similar energy levels on a molecular basis, also implying that the glassy form is extremely stable. In order to induce crystallization, it must be heated to temperatures above 1200°C for long periods of time.
Although pure silica is a perfectly viable glass and glass fiber, it must be worked with at very high temperatures which is a drawback unless its specific chemical properties are needed. It is usual to introduce impurities into the glass in the form of other materials, to lower its working temperature. These materials also impart various other properties to the glass which may be beneficial in different applications. The first type of glass used for fiber was soda-lime glass or A glass. It was not very resistant to alkali.
A new type, E-glass was formed that is alkali free (< 2%) and is an alumino-borosilicate glass. This was the first glass formulation used for continuous filament formation. E-glass still makes up most of the fiberglass production in the world. Its particular components may differ slightly in percentage, but must fall within a specific range. The letter E is used because it was originally for electrical applications. S-glass is a high strength formulation for use when tensile strength is the most important property. C-glass was developed to resist attack from chemicals, mostly acids which destroy E-glass.
Since E-glass does not really melt but soften, the softening point is defined as, “the temperature at which a 0.55 – 0.77 mm diameter fiber 9.25 inches long, elongates under its own weight at 1 mm/min when suspended vertically and heated at the rate of 5°C per minute”. The strain point is reached when the glass has a viscosity of 1014.5 poise. The annealing point, which is the temperature where the internal stresses are reduced to an acceptable commercial limit in 15 minutes. The viscosity at this point should be 1013 poise.
Properties
Glass fibers are useful because of their high ratio of surface area to weight. However, the increased surface makes them much more susceptible to chemical attack.
Glass strengths are usually tested and reported for "virgin" fibers which have just been manufactured. The freshest, thinnest fibers are the strongest and this is thought to be due to the fact that it is easier for thinner fibers to bend. The more the surface is scratched, the less the resulting tenacity is. Because glass has an amorphous structure, its properties are the same along the fiber and across the fiber. Humidity is an important factor in the tensile strength. Moisture is easily adsorbed, and can worsen microscopic cracks and surface defects, and lessen tenacity. In contrast to carbon fiber, glass can undergo more elongation before it breaks.
The viscosity of the molten glass is very important for manufacturing success. During drawing (pulling of the glass to reduce fiber circumference) the viscosity should be relatively low. If it is too high the fiber will break during drawing, however if it is too low the glass will form droplets rather than drawing out into fiber.
Manufacturing Processes
There are two main types of glass fiber manufacture and two main types of glass fiber product. First, fiber is made either from a direct melt process or a marble remelt process. Both start with the raw materials in solid form. The materials are mixed together and melted in a furnace. Then, for the marble process, the molten material is sheared and rolled into marbles which are cooled and packaged. The marbles are taken to the fiber manufacturing facility where they are inserted into a can and remelted. The molten glass is extruded to the bushing to be formed into fiber. In the direct melt process, the molten glass in the furnace goes right to the bushing for formation.
The bushing plate is the most important part of the machinery. This is a small metal furnace containing nozzles for the fiber to be formed through. It is almost always made of platinum alloyed with rhodium for durability. Platinum is used because the glass melt has a natural affinity for wetting it. When bushings were first used they were 100% platinum and the glass wetted the bushing so easily it ran under the plate after exiting the nozzle and accumulated on the underside. Also, due to its cost and the tendency to wear, the platinum was alloyed with rhodium. In the direct melt process, the bushing serves as a collector for the molten glass. It is heated slightly to keep the glass at the correct temperature for fiber formation. In the marble melt process, the bushing acts more like a furnace as it melts more of the material.
The bushings are what make the capital investment in fiber glass production expensive. The nozzle design is also critical. The number of nozzles ranges from 200 to 4000 in multiples of 200. The important part of the nozzle in continuous filament manufacture is the thickness of its walls in the exit region. It was found that inserting a counterbore here reduced wetting. Today, the nozzles are designed to have a minimum thickness at the exit. The reason for this is that as glass flows through the nozzle it forms a drop which is suspended from the end. As it falls, it leaves a thread attached by the meniscus to the nozzle as long as the viscosity is in the correct range for fiber formation. The smaller the annular ring of the nozzle or the thinner the wall at exit, the faster the drop will form and fall away, and the lower its tendency to wet the vertical part of the nozzle. The surface tension of the glass is what influences the formation of the meniscus. For E-glass it should be around 400 mN per m.
The attenuation (drawing) speed is important in the nozzle design. Although slowing this speed down can make coarser fiber, it is uneconomic to run at speeds for which the nozzles were not designed.
In the continuous filament process, after the fiber is drawn, a size is applied. This size helps protect the fiber as it is wound onto a bobbin. The particular size applied relates to end-use. While some sizes are processing aids, others make the fiber have an affinity for a certain resin, if the fiber is to be used in a composite. Size is usually added at 0.5–2.0% by weight. Winding then takes place at around 1000 m per min.
In staple fiber production, there are a number of ways to manufacture the fiber. The glass can be blown or blasted with heat or steam after exiting the formation machine. Usually these fibers are made into some sort of mat. The most common process used is the rotary process. Here, the glass enters a rotating spinner, and due to centrifugal force is thrown out horizontally. The air jets pushes it down vertically and binder is applied. Then the mat is vacuumed to a screen and the binder is cured in the oven.
End uses for regular fiber glass are mats, insulation, reinforcement, heat resistant fabrics, corrosion resistant fabrics and high strength fabrics.
COMPOSITE DIRECTORY
HEALTH ISSUES
In the 1950's, when some of the health effects from asbestos were becoming apparent, fiberglass began to be used as a substitute for these carcinogenic fibers. Due similarities in shape and properties between the two materials, fiberglass was able to effectively replace asbestos in many applications. However, it also raised similar concerns.
As of 2006, researchers and health organizations generally agree that fiberglass is an irritant. Skin irritation is generally associated with thick fibers which can be found in insulation wools and filamentous glass. Fiberglass may also cause irritation of the eyes and throat. If the exposure is sufficient, fiberglass may cause irritation dermatitis and difficulty in breathing, which will generally cease when the exposure ends.
Larger continuous fibers, with diameters greater than 3µm (micrometers) and length greater than 10µm have been designated by the American Conference of Governmental Industrial Hygienists (ACGIH) as an "A4" substance, meaning "Not Classifiable as a Human Carcinogen". This means not that fiberglass is definitely safe, but that there is insufficient data to draw a conclusion either way about a hypothetical carcinogenic potential. Glass wool, with fiber diameters down to 0.05µm and lengths greater than 1µm is currently classified by ACGIH as an "A3" substance. This classifies the glass wool as an animal carcinogen, but indicates that the dose and the routes of exposure of the animal studies are not considered to be comparable to worker or consumer exposure.
LINKS:
|
||||||||||
|
This
website is Copyright
© 1999 & 2013 Max Energy Ltd. The name Solar Navigator are
the bluebird
logo
|
||||||||||