|
KEVLAR
|
|||||||||||
|
Kevlar is the DuPont Company's brand name for material made out of synthetic fiber of poly-paraphenylene terephthalamide which is constructed of para-aramid fibers that the company claims is five times stronger than the same weight of steel, while being lightweight, flexible and comfortable. It is also very heat resistant and decomposes above 400 įC without melting. It was invented by Stephanie Kwolek of DuPont from research into high performance polymers, and patented by her in 1966 and first marketed in 1971. Kevlar is a registered trademark of E.I. du Pont de Nemours and Company. Originally intended to replace the steel belts in tires, it is probably the most well known name in soft armor such as bulletproof vests. It is also used in extreme sports equipment, high-tension drumhead applications, animal handling protection, composite aircraft construction, fire suits, yacht sails, as an asbestos replacement, sometimes in loudspeaker cones, and recently, even in R/C model helicopter blades.
Kevlar body armour - bullet proof vest
When this polymer is spun in the same way that a spider spins a web, the resulting commercial para-aramid fiber has tremendous strength, and is heat and cut resistant. Para-aramid fibers do not rust or corrode, and their strength is unaffected by immersion in water. When woven together, they form a good material for mooring lines and other underwater objects. However, unless specially waterproofed, para-aramid fiberís ability to stop bullets and other projectiles is degraded when wet.
Properties
Kevlar is a processed polyparaphenylene terephthalamide that is an aramid consisting of long polymeric chains with a parallel orientation. Kevlar derives a portion of its improved strength from inter-molecular hydrogen bonds formed between the carbonyl groups and protons on neighboring polymer chains and the partial pi stacking of the benzenoid aromatic stacking interactions between stacked strands. These interactions have a greater influence on Kevlar than van der Waals interactions and chain length that typicaly influence the properties of other synthetic polymers and fibers like Dyneema. In addition the presence of salts and certain other impurities, especially calcium, could interfere with the strand interactions and caution is used to avoid inclusion in its production. Kevlar's structure consists of relatively rigid molecules, which tend to form mostly planar sheet-like structures that have similarities to silk protein.
Poly-paraphenylene terephthalamide intermolecular hydrogen bonding
These properties result in its high mechanical strength and additionally kevlar's remarkable heat resistance. The degree of unsaturated carbons (the ratio of carbon to hydrogen atoms) is quite high, and decreases kevlar's flammability. Kevlar molecules have polar groups accessible for hydrogen bonding. Water that enters the interior of the fiber can take the place of bonding between molecules and reduce the material's strength, while the available groups at the surface lead to good wetting properties. This is important for bonding the fibers to other types of polymer, forming composite material. This same property also makes the fibers feel more natural and "sticky" compared to nonpolar polymers like polyethylene.
There are three common grades of Kevlar: Kevlar, Kevlar 29, and Kevlar 49. Kevlar is typically used as reinforcements in tires and other rubber mechanical goods. Kevlar 29 is used in industrial applications such as cables, asbestos replacement, brake lines, body armor (knife, bullet, etc). Kevlar 49 is considered to have the greatest tensile strength of all the aramids, it is used in applications such as plastic reinforcement for boat hulls, airplanes, and bikes.
In structural applications, Kevlar fibers can be bonded to one another or to other materials to form a composite.
Kevlar's main weaknesses are that it decomposes under alkaline conditions or when exposed to chlorine. While it can have a great tensile strength, sometimes in excess of 4.0 GPa, like all fibers it tends to buckle in compression.
Production
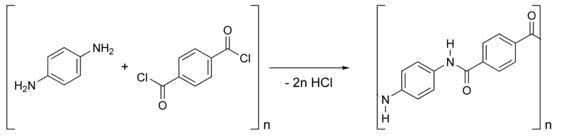
Kevlar is synthesized from the monomers 1,4-phenyl-diamine (para-phenylenediamine) and terephthaloyl chloride. The result is a polymeric aromatic amide (aramid) with alternating benzene rings and amide groups. When they are produced, these polymer strands are aligned randomly. To make Kevlar, they are dissolved and spun, the combination of lyotropic liquid-crystalline behaviour and mechanical drawing causing the polymer chains to orientate in the direction of the fibre.
Kevlar has a high price, in part, due to the difficulties arising from the use of concentrated sulfuric acid in its manufacture. These harsh conditions are needed to keep the highly insoluble polymer in solution during synthesis and spinning.
COMPOSITE DIRECTORY
LINKS
Working with Carbon fibre for Robotics and R/C Aircraft Building a fibreglass fishing boat Working with Carbon Fiber for Robotics and R/C Aircraft Japan Carbon Fibre Manufacturers Association (English) Carbon fibre page from the Department of Polymer Science at USM BMW's use of carbon fiber reinforced plastics
|
|||||||||||
|
This
website is Copyright
© 1999 & 2013 Max Energy Ltd. The name Solar Navigator are
the bluebird
logo
|
|||||||||||