|

ENERGY
TRADING 24 JANUARY 2013
LONDON, ENGLAND - The UK and Ireland today pledged to work together to secure economic benefits for both countries through renewable
energy trading.
A memorandum of understanding was signed by UK Energy Secretary Ed Davey and his Irish counterpart Pat Rabbitte in Dublin. The two
countries have agreed to investigate ways to achieve more cost efficient uses of resources, drive down deployment costs and reduce
dependence on fossil fuels.
It is hoped that through the MoU, an inter-governmental agreement could be signed in 2014 in time for potential projects to start
exporting wind energy from Ireland to Britain by 2020.
Rabbitte said today that Ireland has “the potential to generate far more wind energy than we could consume domestically. The opportunity
to export this green power presents an opportunity for employment growth and export earnings which we must seize if we can.”
Davey said that trading power with Ireland “could increase the amount
of green power in our energy mix and potentially bring down costs for UK consumers”.
“Connecting supplies between countries also gives us the opportunity
to feed more electricity from renewable sources into the grid at any one time, so that we can cut down on the amount of expensive
old-school fossil fuels we have to import.”
He added that he hoped interconnectors between countries would eventually be developed across Europe.
"We can’t control the international price of gas, but we know exactly how cost-effective
wind energy is."
www.renewableenergyworld.com/uk
ireland sign renewable energy trading January 25 2013
We
all know what energy is. We need food to energize our bodies. We need
electricity in the home and for factories. We need petrol for our cars
and gas or oil for our central heating. That's easy to understand: we
get hungry and cold. But, most importantly, we need the Sun
to keep pouring those glorious rays upon us, or we'll all die -
because ultimately, all life on Earth is dependent on atomic hydrogen fusion.
Of course we don't see it like that, we just enjoy it when the Sun
comes out. Make a note here that fusion is the opposite of fission,
which is when a nuclear missile explodes.

Sea snake wave generator,
is it cost effective?
But
in physics, we must look at things differently. Energy (Ancient Greek:
νέργεια energeia "activity,
operation") is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical
systems. Since work is defined as a force acting through a distance (a length of space), energy is always equivalent to the ability to exert pulls or pushes against the basic forces of nature, along a path of a certain length.
The total energy contained in an object is linked to its mass, and energy (like mass), cannot be created or destroyed.
Even though it might seem that way to the casual observer. When matter (ordinary material particles) is changed into energy (such as energy of motion, or into radiation), the mass of the system does not change through the transformation process. However, there may be mechanistic limits as to how much of the matter in an object may be changed into other types of energy and thus into work, on other systems. Energy, like mass, is a scalar physical quantity. In the International System of Units (SI), energy is measured in joules, but in many fields other units, such as kilowatt-hours and kilocalories, are
used on an everyday basis. All of these units translate to units of work, which is always defined in terms of forces and the distances that the forces act through.
A system can transfer energy to another system by simply transferring matter to it (since matter is equivalent to energy, in accordance with its mass). However, when energy is transferred by means other than matter-transfer, the transfer produces changes in the second system, as a result of work done on it. This work manifests itself as the effect of force(s) applied through distances within the target system. For example, a system can emit energy to another by transferring (radiating) electromagnetic energy, but this creates forces upon the particles that absorb the radiation. Similarly, a system may transfer energy to another by physically impacting it, but in that case the energy of motion in an object, called kinetic energy, results in forces acting over distances (new energy) to appear in another object that is struck. Transfer of thermal energy by heat occurs by both of these mechanisms: heat can be transferred by electromagnetic radiation, or by physical contact in which direct particle-particle impacts transfer kinetic energy.

A
different kind of wave actuated generator
Energy may be stored in systems without being present as matter, or as kinetic or electromagnetic energy. Stored energy is created whenever a particle has been moved through a field it interacts with (requiring a force to do so), but the energy to accomplish this is stored as a new position of the particles in the field—a configuration that must be "held" or fixed by a different type of force (otherwise, the new configuration would resolve itself by the field pushing or pulling the particle back toward its previous position). This type of energy "stored" by force-fields and particles that have been forced into a new physical configuration in the field by doing work on them by another system, is referred to as potential energy. A simple example of potential energy is the work needed to lift an object in a gravity field, up to a support. Each of the basic forces of nature is associated with a different type of potential energy, and all types of potential energy (like all other types of energy) appears as system mass, whenever present. For example, a compressed spring will be slightly more massive than before it was compressed. Likewise, whenever energy is transferred between systems by any mechanism, an associated mass is transferred with it.
Any form of energy may be transformed into another form. For example, all types of potential energy are converted into kinetic energy when the objects are given freedom to move to different position (as for example, when an object falls off a support). When energy is in a form other than thermal energy, it may be transformed with good or even perfect efficiency, to any other type of energy, including electricity or production of new particles of matter. With thermal energy, however, there are often limits to the efficiency of the conversion to other forms of energy, as described by the second law of thermodynamics.
In all such energy transformation processes, the total energy remains the same, and a transfer of energy from one system to another, results in a loss to compensate for any gain. This principle, the conservation of energy, was first postulated in the early 19th century, and applies to any isolated system. According to Noether's theorem, the conservation of energy is a consequence of the fact that the laws of physics do not change over
time.
Although the total energy of a system does not change with time, its value may depend on the frame of reference. For example, a seated passenger in a moving airplane has zero kinetic energy relative to the airplane, but non-zero kinetic energy (and higher total energy) relative to the Earth.
Hydroelectric
power generation Hoover Dam
Conservation of
energy
Energy is subject to the law of conservation of energy. According to this law, energy can neither be created (produced) nor destroyed by itself. It can only be transformed.
Most kinds of energy (with gravitational energy being a notable
exception) are subject to strict local conservation laws as well. In this case, energy can only be exchanged between adjacent regions of space, and all observers agree as to the volumetric density of energy in any given space. There is also a global law of conservation of energy, stating that the total energy of the universe cannot change; this is a corollary of the local law, but not vice
versa. Conservation of energy is the mathematical consequence of translational symmetry of time (that is, the indistinguishability of time intervals taken at different
time) - see Noether's theorem.
According to Conservation of energy the total inflow of energy into a system must equal the total outflow of energy from the system, plus the change in the energy contained within the system.
This law is a fundamental principle of physics. It follows from the translational symmetry of time, a property of most phenomena below the cosmic scale that makes them independent of their locations on the time coordinate. Put differently, yesterday, today, and tomorrow are physically indistinguishable.
This is because energy is the quantity which is canonical conjugate to time. This mathematical entanglement of energy and time also results in the uncertainty principle - it is impossible to define the exact amount of energy during any definite time interval. The uncertainty principle should not be confused with energy conservation - rather it provides mathematical limits to which energy can in principle be defined and measured.
Chevron
on vehicle technology Energy and the laws of
motion n classical mechanics, energy is a conceptually and mathematically useful property, as it is a conserved quantity. Several formulations of mechanics have been developed using energy as a core concept.
The Hamiltonian
The total energy of a system is sometimes called the Hamiltonian, after William Rowan Hamilton. The classical equations of motion can be written in terms of the Hamiltonian, even for highly complex or abstract systems. These classical equations have remarkably direct analogs in nonrelativistic quantum
mechanics.
The Lagrangian
Another energy-related concept is called the Lagrangian, after Joseph Louis Lagrange. This is even more fundamental than the Hamiltonian, and can be used to derive the equations of motion. It was invented in the context of classical mechanics, but is generally useful in modern physics. The Lagrangian is defined as the kinetic energy minus the potential energy.
Usually, the Lagrange formalism is mathematically more convenient than the Hamiltonian for non-conservative systems (such as systems with friction).
Noether's Theorem Noether's (first) theorem (1918) states that any differentiable symmetry of the action of a physical system has a corresponding conservation law.
Noether's theorem has become a fundamental tool of modern theoretical physics and the calculus of variations. A generalization of the seminal formulations on constants of motion in Lagrangian and Hamiltonian mechanics (1788 and 1833, respectively), it does not apply to systems that cannot be modeled with a Lagrangian; for example, dissipative systems with continuous symmetries need not have a corresponding conservation law.

A
nuclear (atomic fission) power station in France
uses
plutonium rods near critical mass (slow release) to generate heat
for
steam turbine powered generators
Energy and thermodynamics
Internal
energy Internal energy is the sum of all microscopic forms of energy of a system. It is the energy needed to create the system. It is related to the potential energy, e.g., molecular structure, crystal structure, and other geometric aspects, as well as the motion of the particles, in form of kinetic energy. Thermodynamics is chiefly concerned with changes in internal energy and not its absolute value, which is impossible to determine with thermodynamics
alone.
The first law of thermodynamics
The first law of thermodynamics asserts that energy is
conserved and that heat flow is a form of energy transfer. For homogeneous systems, with a well-defined temperature and pressure, a commonly used corollary of the first law is that, for a system subject only to pressure forces and heat transfer (e.g., a cylinder-full of gas), the differential change in the internal energy of the system (with a gain in energy signified by a positive quantity) is given as 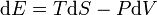 , ,
where the first term on the right is the heat transferred into the system, expressed in terms of temperature T and entropy S (in which entropy increases and the change dS is positive when the system is heated), and the last term on the right hand side is identified as work done on the system, where pressure is P and volume V (the negative sign results since compression of the system requires work to be done on it and so the volume change, dV, is negative when work is done on the system).
|

|

|
|
Dirty
oil energy |
Clean
solar energy |
This equation is highly specific, ignoring all chemical, electrical, nuclear, and gravitational forces, effects such as advection of any form of energy other than heat and pV-work. The general formulation of the first law (i.e., conservation of energy) is valid even in situations in which the system is not homogeneous. For these cases the change in internal energy of a closed system is expressed in a general form by
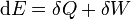
where 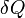 is the heat supplied to the system and
is the heat supplied to the system and 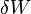 is the work applied to the system.
is the work applied to the system.
Equipartition of energy
The energy of a mechanical harmonic oscillator (a mass on a spring) is alternatively kinetic and potential. At two points in the oscillation cycle it is entirely kinetic, and alternatively at two other points it is entirely potential. Over the whole cycle, or over many cycles, net energy is thus equally split between kinetic and potential. This is called equipartition principle; total energy of a system with many degrees of freedom is equally split among all available degrees of freedom.
This principle is vitally important to understanding the behavior of a quantity closely related to energy, called entropy. Entropy is a measure of evenness of a distribution of energy between parts of a system. When an isolated system is given more degrees of freedom (i.e., given new available energy states that are the same as existing states), then total energy spreads over all available degrees equally without distinction between "new" and "old" degrees. This mathematical result is called the second law of thermodynamics
Wind
turbines in Southern California Quantum
mechanics
In quantum mechanics energy is defined in terms of the energy operator as a time derivative of the wave function. The Schrödinger equation equates the energy operator to the full energy of a particle or a system. In results can be considered as a definition of measurement of energy in quantum mechanics.
The Schrödinger equation describes the space- and time-dependence of slow changing (non-relativistic) wave function of quantum systems. The solution of this equation for bound system is discrete (a set of permitted states, each characterized by an energy level) which results in the concept of quanta. In the solution of the Schrödinger equation for any oscillator (vibrator) and for electromagnetic waves in a vacuum, the resulting energy states are related to the frequency by the Planck equation (where is the Planck's constant and the frequency). In the case of electromagnetic wave these energy states are called quanta of light or photons.
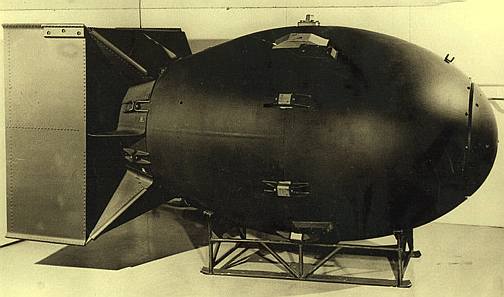
A
nuclear (atomic fission) bomb brings plutonium to critical mass
quickly
this
bomb was dropped on Nagasaki 9th August 1945 - man being irresponsible
Relativity When calculating kinetic energy (work to accelerate a mass from zero speed to some finite speed) relativistically - using Lorentz transformations instead of Newtonian mechanics, Einstein discovered an unexpected by-product of these calculations to be an energy term which does not vanish at zero speed. He called it rest mass energy - energy which every mass must possess even when being at rest. The amount of energy is directly proportional to the mass of body:
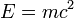 ,
where: ,
where:
m is the mass,
c is the speed of light in vacuum,
E is the rest mass energy.
For example, consider electron-positron annihilation, in which the rest mass of individual particles is destroyed, but the inertia equivalent of the system of the two particles (its invariant mass) remains (since all energy is associated with mass), and this inertia and invariant mass is carried off by photons which individually are
mass-less, but as a system retain their mass. This is a reversible process - the inverse process is called pair creation - in which the rest mass of particles is created from energy of two (or more) annihilating photons. In this system the matter (electrons and positrons) is destroyed and changed to non-matter energy (the photons). However, the total system mass and energy do not change during this interaction.
In general relativity, the stress-energy tensor serves as the source term for the gravitational field, in rough analogy to the way mass serves as the source term in the non-relativistic Newtonian
approximation.
It is not uncommon to hear that energy is "equivalent" to mass. It would be more accurate to state that every energy has an inertia and gravity equivalent, and because mass is a form of energy, then mass too has inertia and gravity associated with it.

A
nuclear detonation: Operation Castle Romeo 1954
Energy and life
Bioenergetics
- Basic overview of energy and human life. Any living organism relies on an external source of energy—radiation from the Sun in the case of green plants; chemical energy in some form in the case of animals—to be able to grow and reproduce. The daily 1500–2000 Calories (6–8 MJ) recommended for a human adult are taken as a combination of oxygen and food molecules, the latter mostly carbohydrates and fats, of which glucose (C6H12O6) and stearin (C57H110O6) are convenient examples. The food molecules are oxidised to carbon dioxide and water in the mitochondria
C6H12O6 + 6O2 -> 6CO2 + 6H2O
C57H110O6 + 81.5O2 -> 57CO2 + 55H2O
and some of the energy is used to convert ADP into ATP
ADP + HPO42− -> ATP + H2O
The rest of the chemical energy in the carbohydrate or fat is converted into heat: the ATP is used as a sort of "energy currency",
and some of the chemical energy it contains when split and reacted with water, is used for other metabolism (at each stage of a metabolic pathway, some chemical energy is converted into heat). Only a tiny fraction of the original chemical energy is used for
work:
-
gain in kinetic energy of a sprinter during a 100 m race: 4 kJ
-
gain in gravitational potential energy of a 150 kg weight lifted through 2 metres: 3kJ
-
Daily food intake of a normal adult: 6–8 MJ
It would appear that living organisms are remarkably inefficient (in the physical sense) in their use of the energy they receive (chemical energy or radiation), and it is true that most real machines manage higher efficiencies. In growing organisms the energy that is converted to heat serves a vital purpose, as it allows the organism tissue to be highly ordered with regard to the molecules it is built from. The second law of thermodynamics states that energy (and matter) tends to become more evenly spread out across the universe: to concentrate energy (or matter) in one specific place, it is necessary to spread out a greater amount of energy (as heat) across the remainder of the universe ("the
surroundings"). Simpler organisms can achieve higher energy efficiencies than more complex ones, but the complex organisms can occupy ecological niches that are not available to their simpler brethren. The conversion of a portion of the chemical energy to heat at each step in a metabolic pathway is the physical reason behind the pyramid of biomass observed in ecology: to take just the first step in the food chain, of the estimated 124.7 Pg/a of carbon that is fixed by photosynthesis, 64.3 Pg/a (52%) are used for the metabolism of green
plants, i.e. reconverted into carbon dioxide and heat.

A
nuclear detonation: 2nd World War, Nagasaki, Japan 1945
Measurement
Because energy is defined as the ability to do work on objects, there is no absolute measure of energy. Only the transition of a system from one state into another can be defined and thus energy is measured in relative terms. The choice of a baseline or zero point is often arbitrary and can be made in whatever way is most convenient for a problem.
Methods
The methods for the measurement of energy often deploy methods for the measurement of still more fundamental concepts of science, namely mass, distance, radiation, temperature, time, electric charge and electric current.
Conventionally the technique most often employed is calorimetry, a thermodynamic technique that relies on the measurement of temperature using a thermometer or of intensity of radiation using a
bolometer.

A
Trident II missile with a nuclear tipped warhead Units of energy
Throughout the history of science, energy has been expressed in several different units such as ergs and calories. At present, the accepted unit of measurement for energy is the SI unit of energy, the joule. In addition to the joule, other units of energy include the kilowatt hour (kWh) and the British thermal unit (Btu). These are both larger units of energy. One kWh is equivalent to exactly 3.6 million joules, and one Btu is equivalent to about 1055
joules.
Energy density
Energy density is a term used for the amount of useful energy stored in a given system or region of space per unit volume.
For fuels, the energy per unit volume is sometimes a useful parameter. In a few applications, comparing, for example, the effectiveness of hydrogen fuel to gasoline it turns out that hydrogen has a higher specific energy than does gasoline, but, even in liquid form, a much lower energy density.
Homemade
windmill generator Forms of
energy
Heat, a form of energy, is partly potential energy and partly kinetic
energy. In the context of physical sciences, several forms of energy have been defined. These include:
-
Thermal energy, thermal energy in transit is called heat
-
Chemical energy
-
Electric energy
-
Radiant energy, the energy of electromagnetic radiation
-
Nuclear energy
-
Magnetic energy
-
Elastic energy
-
Sound energy
-
Mechanical energy
-
Luminous energy
-
Mass (E=mc²)
These forms of energy may be divided into two main groups; kinetic energy and potential energy. Other familiar types of energy are a varying mix of both potential and kinetic energy.
Energy may be transformed between these forms, some with 100% energy conversion efficiency and others with less. Items that transform between these forms are called transducers.

Wind turbines are the start of a renewable dawn, hope for our energy future
The above list of the known possible forms of energy is not necessarily complete. Whenever physical scientists discover that a certain phenomenon appears to violate the law of energy conservation, new forms may be added, as is the case with dark energy, a hypothetical form of energy that permeates all of space and tends to increase the rate of expansion of the universe.
Classical mechanics distinguishes between potential energy, which is a function of the position of an object, and kinetic energy, which is a function of its movement. Both position and movement are relative to a frame of reference, which must be specified: this is often (and originally) an arbitrary fixed point on the surface of the Earth, the terrestrial frame of reference. It has been attempted to categorize all forms of energy as either kinetic or potential: this is not incorrect, but neither is it clear that it is a real simplification, as Feynman points out:
These notions of potential and kinetic energy depend on a notion of length scale. For example, one can speak of macroscopic potential and kinetic energy, which do not include thermal potential and kinetic energy. Also what is called chemical potential energy is a macroscopic notion, and closer examination shows that it is really the sum of the potential and kinetic energy on the atomic and subatomic scale. Similar remarks apply to nuclear "potential" energy and most other forms of energy. This dependence on length scale is non-problematic if the various length scales are decoupled, as is often the case ... but confusion can arise when different length scales are coupled, for instance when friction converts macroscopic work into microscopic thermal energy.
Niagara
Falls - a vast body moving of water = potential energy
Transformations of energy
One form of energy can often be readily transformed into another with the help of a device- for instance, a battery, from chemical energy to
electric energy; a dam: gravitational potential energy to kinetic energy of moving water (and the blades of a turbine) and ultimately to electric energy through an electric generator. Similarly, in the case of a chemical explosion, chemical potential energy is transformed to kinetic energy and thermal energy in a very short time. Yet another example is that of a pendulum. At its highest points the kinetic energy is zero and the gravitational potential energy is at maximum. At its lowest point the kinetic energy is at maximum and is equal to the decrease of potential energy. If one (unrealistically) assumes that there is no friction, the conversion of energy between these processes is perfect, and the pendulum will continue swinging forever.
Energy gives rise to weight when it is trapped in a system with zero momentum, where it can be weighed. It is also equivalent to mass, and this mass is always associated with it. Mass is also equivalent to a certain amount of energy, and likewise always appears associated with it, as described in mass-energy equivalence. The formula E = mc², derived by Albert Einstein (1905) quantifies the relationship between rest-mass and rest-energy within the concept of special relativity. In different theoretical frameworks, similar formulas were derived by J. J. Thomson (1881), Henri Poincaré (1900), Friedrich Hasenöhrl (1904) and others (see Mass-energy equivalence#History for further information).

Germany
wants to rid itself of the dangers presented
by nuclear power stations
Matter may be destroyed and converted to energy (and vice versa), but mass cannot ever be destroyed; rather, mass remains a constant for both the matter and the energy, during any process when they are converted into each other. However, since is extremely large relative to ordinary human scales, the conversion of ordinary amount of matter (for example, 1 kg) to other forms of energy (such as heat, light, and other radiation) can liberate tremendous amounts of energy (~ joules = 21 megatons of TNT), as can be seen in nuclear reactors and nuclear weapons. Conversely, the mass equivalent of a unit of energy is minuscule, which is why a loss of energy (loss of mass) from most systems is difficult to measure by weight, unless the energy loss is very large. Examples of energy transformation into matter (i.e., kinetic energy into particles with rest mass) are found in high-energy nuclear physics.
Transformation of energy into useful work is a core topic of thermodynamics. In nature, transformations of energy can be fundamentally classed into two kinds: those that are thermodynamically reversible, and those that are thermodynamically irreversible. A reversible process in thermodynamics is one in which no energy is dissipated (spread) into empty energy states available in a volume, from which it cannot be recovered into more concentrated forms (fewer quantum states), without degradation of even more energy. 
Pylons
are a clean, quiet and relatively safe way of distributing energy A reversible process is one in which this sort of dissipation does not happen. For example, conversion of energy from one type of potential field to another, is reversible, as in the pendulum system described above. In processes where heat is generated, quantum states of lower energy, present as possible excitations in fields between atoms, act as a reservoir for part of the energy, from which it cannot be recovered, in order to be converted with 100% efficiency into other forms of energy. In this case, the energy must partly stay as heat, and cannot be completely recovered as usable energy, except at the price of an increase in some other kind of heat-like increase in disorder in quantum states, in the universe (such as an expansion of matter, or a randomization in a crystal).
As the universe evolves in time, more and more of its energy becomes trapped in irreversible states (i.e., as heat or other kinds of increases in disorder). This has been referred to as the inevitable thermodynamic heat death of the universe. In this heat death the energy of the universe does not change, but the fraction of energy which is available to do work through a heat engine, or be transformed to other usable forms of energy (through the use of generators attached to heat engines), grows less and less.
Is that likely to happen in our lifetime? No chance, but it is a
chilling thought.
     
     
     
ENERGY
GENERATING-DISTRIBUTION UTILITIES
E-ON
wave energy - sea snake LINKS: "Work, Power, Kinetic Energy" Global Mapping of Terrestrial Primary Productivity and Light-Use Efficiency Light and Matter Time
Invariance Light and Matter Energy
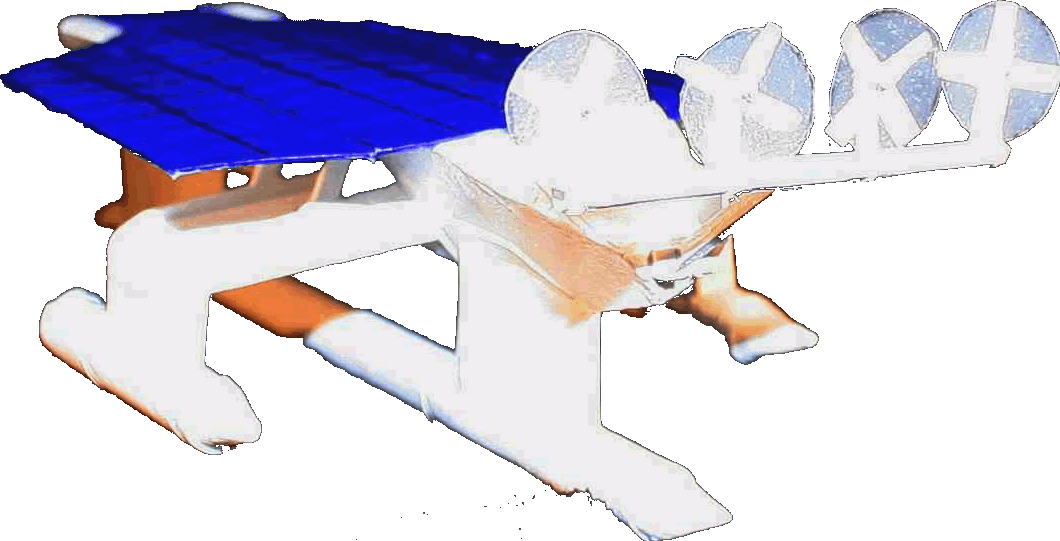
The
Elizabeth Swan is
a zero carbon ship powered by solar panels and wind turbines.
This
robot ship may be manned for the Kulo
Luna environmental adventure story.
|































