|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Nelson worked for a time at the Jewellers shop his family owned in Newhaven, Sussex, making rings and other special orders, doing repairs, etc. In March of 1981 he was elected a full member of the Guild of Master Crafstmen for his work as a goldsmith. This intricate and rewarding craft was of enormous benefit when it came to producing accurate small models of his vehicle designs.
The Designer
We would like to offer our congratulations to Sarah Ingram of Spectrum Fine Jewellery, Battle, East Sussex, for her steadfast achievements and completion of a five year apprenticeship as reported at the London Metropolitan Website below.
London Metropolitan University - Announcements / In the News / December 2004
Battle Observer 10 December 2004: Reported the news that Sarah Ingram was granted the freedom of Goldsmiths at the Goldsmiths Hall after completing a five year apprenticeship. Ms Ingram studied jewellery design at London Metropolitan University. Sarah's father, Keith Ingram is a member of the Institute of Professional Goldsmiths as a diamond mounter.
CONTACT: Keith Ingram Spectrum Fine Jewellery Limited, 46 High Street, Battle, East Sussex Telephone: 01424 774404
Wedding rings
The Institute of Professional Goldsmiths is the top qualifying body in the manufacturing gold and silver trade. Members of the Institute have been engaged in the craft for a minimum of fifteen years, and their work has been judged by their peers to be of the highest quality.
All members are practising craftsmen, recognised and respected for their high standard of professional accomplishment. The Institute is devoted exclusively to protecting and maintaining the very highest standards of craftsmanship demanded by retail outlets. It develops and assists positive and acceptable methods of training as well as good professional practice.
IPO Home | About IPO | Members | Crafts | Contact | News
The
Worshipful Company of Goldsmiths, more commonly known as the
Goldsmiths' Company, is one of the Twelve Great Livery Companies of
the City of London and received its first royal charter in 1327.
Goldsmiths Home | Goldsmiths Company | Assay Office | Training
JEWELLERY (JEWELRY us spelling)
Jewellery comprises ornamental objects worn by persons, typically made with gems and precious metals. Costume jewellery is made from less valuable materials. However, jewellery can and has been made out of almost every kind of material.
The word is derived from the word "jewel", which was anglicised from the Old French "jouel" in around the 13th century. Further tracing leads back to the Latin word "jocale", meaning plaything.
Some cultures have a practice of keeping large amounts of wealth stored in the form of jewellery. Jewellery can also be symbolic, as in the case of Christians wearing a crucifix in the form of jewellery, or, as is the case in many Western cultures, married people wearing a wedding ring.
Jewellery in various forms has been made and worn by both sexes in almost every (if not every) human culture, on every inhabited continent. Personal adornment seems to be a basic human tendency.
Types
Materials and methods
Jewellery, particularly when made with precious materials, is generally considered valuable and desirable. A variety of precious gemstones, coins or other precious items can be used, often set into precious metals. Common metals used for jewellery include gold, platinum or silver. Most gold alloys used in jewellery range from 10K to 22K gold, while platinum alloys range from 900 (90% pure) to 950 (95.0% pure). The silver used in jewellery is often sterling silver.
Jeweller working on a ring
Common gemstones that are used include diamonds, rubies, sapphires, emeralds, and opals. Dozens more are also commonly used.
Other commonly used materials include glass, such as fused glass or enamel; wood, often carved or turned; shells and other natural animal substances such as bone and ivory; and natural clay and plasticine clays, such as polymer clay.
Beads are commonly used in jewellery. These may be made of many different substances including glass, gemstones, wood, shells, clay and polymer clay. Beaded jewellery commonly encompasses necklaces, bracelets, earrings, and belts. Beads may be large or small. The smallest type of beads commonly used are known as seed beads; these are the beads used for the "woven" style of beaded jewellery.
Timeline
This is a timeline of jewellery production from the first uses of metal in history to the Renaissance.
7000 BC - Uses of copper in Anatolia, Iran and Eastern Europe. 5000 BC - Uses of copper in Egypt. 4000 BC - Smelting technology for copper in Egypt and Iran. 3450 BC - Use of natural zinc/copper alloy in Egypt. 3500 BC - Gold makes an appearance in Egyptian jewellery. 3000 BC - Egypt and Iran makeing simple hammered iron beads 3000 BC - The Middle East employ semi-mass-production 2000 BC - First signs of the swagging technique 2600 BC - Beaded wires began to be used. 2500 BC - Egyptians using copper/lead alloys. 2500 BC - True iron production technology in Near East. 2500 BC - The intentional addition of silver and copper to gold. 2500 BC - Gold wires are characterised by seam lines that follow a spiral path along the wire. 2000 BC - Use of patterned punches 1500 BC - Earplugs and earrings become popular in Egypt. 1400 BC - Egypt Amarna period, using resin and mud for repoussé backing. 1400 BC - Deliberate addition of zinc to copper in Canaan. 1400 BC - Philistines have iron. 1400 BC - Very copper rich gold alloys popular in Egypt. 1000 BC - Persian sheet bronze work 0.05mm thick. 1000 BC - The start of true engraving. 900 BC - The Greeks have iron. 700 BC - World's oldest coinage in Lydia. 575 BC - In Greece, jewellery is still very rare. 500 BC - Hafted hammers were being used in some parts. 500 BC - Iron in use in Britain 400 BC - Greeks using Beeswax for filler in repoussé. 350 BC - Use of combined punches and dies of bronze. 325 BC - Animal or human-headed hoop earrings were popular. 300 BC - Diadems are first seen. 300 BC - Red Coral popular in Celtic jewellery. AD 50 - Start of the Roman period, where addition of silver to gold becomes almost unknown. AD 100 - Sulphur fills hollow gold items throughout the Roman Empire. AD 150 - Tin rings found in Nubia AD 300 - Lead becomes more common in places. AD 400 - Pewter jewellery is made. AD 400 - A shale die is found in Britain. AD 1500 - The Renaissance
Goldsmiths and silversmiths are metalworkers who specializes in working with precious metals, usually to make jewellery and fine decorative utensils of gold, silver, copper, bronze and iron.
Goldsmiths must be skilled in forming metal, through filing, soldering, forging, casting and polishing. Traditionally these skills are passed along through apprenticeships. More recently some schools have begun offering courses in goldsmithing.
At one time, the name was synonymous with banker, since they dealt in gold and had sufficient security for safe storage of valuable items.
Fort Knox - US gold reserves
GOLD
Gold is a chemical element in the periodic table that has the symbol Au (L. aurum) and atomic number 79. A soft, shiny, yellow, dense, malleable, ductile (trivalent and univalent) transition metal, gold does not react with most chemicals but is attacked by chlorine, fluorine and aqua regia. The metal occurs as nuggets or grains in rocks and in alluvial deposits and is one of the coinage metals.
For millennia, gold has served as money and is also used in jewellery, dentistry, and in electronics. Gold forms the basis for a monetary standard used by the International Monetary Fund (IMF) and the Bank for International Settlements (BIS). Its ISO currency code is XAU. Notable characteristics
Gold is a metallic element with a characteristic yellow color, but can also be black or ruby when finely divided, while colloidal solutions are intensely colored and often purple. These colors are the result of gold's plasmon frequency lying in the visible range, which causes red and yellow light to be reflected, and blue light to be absorbed. Only silver colloids exhibit the same interactions with light, albeit at a shorter frequency, making silver colloids yellow in color.
It is the most malleable and ductile metal known; a single gram can be beaten into a sheet of one square metre, or an ounce into 300 square feet. A soft metal, gold will readily form alloys with many other metals. This can be done to increase its strength, or create several exotic colors, sold for instance in the western United States to the tourist trade as "Black Hills" gold. Adding copper yields a redder metal, iron blue, Silver produces green, aluminium purple, platinum metals white, and natural bismuth together with silver alloys produce black. Native gold contains usually eight to ten per cent silver, but often much more — alloys with a silver content over 20% are called electrum. As the amount of silver increases, the color becomes whiter and the specific gravity lower.
Gold is a good conductor of heat and electricity, and is not affected by air and most reagents. Heat, moisture, oxygen, and most corrosive agents have very little chemical effect on gold, making it well-suited for use in coins and jewelry; conversely, halogens will chemically alter gold, and aqua regia dissolves it.
Common oxidation states of gold include +1 (gold(I) or aurous compounds) and +3 (gold(III) or auric compounds). Gold ions in solution are readily reduced and precipitated out as gold metal by the addition of virtually any other metal as the reducing agent. The added metal is oxidized and dissolves allowing the gold to be displaced from solution and be recovered as a solid precipitate.
Recent research undertaken by Frank Reith of the Australian National University shows that microbes play an important role in the formation of gold deposits, transporting and precipitating gold to form grains and nuggets that collect in alluvial deposits.
Applications
Pure gold is too soft for ordinary use and is hardened by alloying with silver, copper, and other metals. Gold and its many alloys are most often used in jewelry, coinage and as a standard for monetary exchange in many countries. Because of its high electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal.
Gold and silver axe, 3rd-2nd millennium BC
History
Gold (Sanskrit jval, Greek χρυσος [khrusos], Latin aurum for "shining dawn", Anglo-Saxon gold, Chinese 金 [jīn],Japanese 金 [kin]) has been known and highly valued since prehistoric times. It may have been the first metal used by humans and was valued for ornamentation and rituals. Egyptian hieroglyphs from as early as 2600 BC describe gold, which king Tushratta of the Mitanni claimed was as "common as dust" in Egypt. Egypt and Nubia had the resources to make them major gold-producing areas for much of history. Gold is also mentioned several times in the Old Testament. The south-east corner of the Black Sea was famed for its gold. Exploitation is said to date from the time of Midas, and this gold was important in the establishment of what is probably the world's earliest coinage in Lydia between 643 and 630 BC.
The European exploration of the Americas was fueled in no small part by reports of the gold ornaments displayed in great profusion by Native American peoples, especially in Central America, Peru, and Colombia.
Gold has long been considered one of the most precious metals, and its value has been used as the standard for many currencies (known as the gold standard) in history. Gold has been used as a symbol for purity, value, royalty, and particularly roles that combine these properties.
Gold in antiquity was relatively easy to obtain geologically; however, 75% of all gold ever produced has been extracted since 1910. It has been estimated that all the gold in the world that has ever been refined would form a single cube 20 m (66 ft) a side. The primary goal of the alchemists was to produce gold from other substances, such as lead — presumably by the interaction with a mythical substance called the philosopher's stone. Although they never succeeded in this attempt, the alchemists promoted an interest in what can be done with substances, and this laid a foundation for today's chemistry. Their symbol for gold was the circle with a point at its center (☉), which was also the astrological symbol, the Egyptian hieroglyph and the ancient Chinese character for the Sun (now 日).
During the 19th century, gold rushes occurred whenever large gold deposits were discovered, including the California, Colorado, Otago, Australia, Witwatersrand, Black Hills, and Klondike gold rushes.
Because of its historically high value, much of the gold mined throughout history is still in circulation in one form or another.
Gold bars or ingots
Value
Like other precious metals, gold is measured by troy weight and by grams. When it is alloyed with other metals the term carat or karat is used to indicate the amount of gold present, with 24 carats being pure gold and lower ratings proportionally less. The purity of a gold bar can also be expressed as a decimal figure ranging from 0 to 1, known as the millesimal fineness, such as 0.995.
The price of gold is determined on the open market, but a procedure known as the Gold Fixing in London, originating in 1919, provides a twice-daily benchmark figure to the industry.
Historically gold was used to back currency in an economic system known as the gold standard in which one unit of currency was equivalent to a certain weight of gold. As part of this system, governments and central banks attempted to control the price of gold by setting values at which they would exchange it for currency. For a long period the United States government set the price of gold at $20.67 per troy ounce ($664.56/kg) but in 1934 the price of gold was set at $35.00 per troy ounce ($1125.27/kg). By 1961 it was becoming hard to maintain this price, and a pool of US and European banks began to act together to defend the price against market forces.
On March 17, 1968, economic circumstances caused the collapse of the gold pool, and a two-tiered pricing scheme was established whereby gold was still used to settle international accounts at the old $35.00 per troy ounce ($1.13/g) but the price of gold on the private market was allowed to fluctuate; this two-tiered pricing system was abandoned in 1975 when the price of gold was left to find its free-market level. Central banks still hold historical gold reserves as a reserve asset although the level has generally been declining. The largest gold depository in the world is that of the U.S. Federal Reserve Bank, in New York.
Since 1968 the price of gold on the open market has ranged widely, with a record high of $850/oz ($27,300/kg) on 21 January 1980, to a low of $252.90/oz ($8,131/kg) on 21 June 1999 (London Fixing). Prices have risen to the $500/oz mark in late 2005, due to a depreciation of the US dollar and inflation due to rising energy costs.In January 2006 the goldprice rose above $555/oz and many observers think that fears of a nose-diving dollar will send the price of gold even much higher.
Gold and the money supply
In January 1959 US M3 money supply was $288.8 billion, and the Official Gold Holdings of the United States was then 17,335.1 Tonnes, or about 557 million ounces (there are 32,150.7 Troy Ounces in a Tonne). That means that in 1959, there were $518 in circulation for every ounce of gold reserves held by the USA. Although the theoretical price should then have been $518 per ounce, the actual price, as fixed under the gold standard was only $35 an ounce.
By August 2005, the US M3 money supply had risen to $9,873.9 billion, whilst at the same time the Official Gold Holdings of the United States had fallen to just 8,133.5 Tonnes, or about 261 million Troy Ounces. This means that today, in 2005, there are $37,831 in circulation for every ounce of gold held by the United States. The above numbers show the falling influence of gold in the monetary system of the world today. Goldbugs believe, or even hope, that one day gold's importance will return as the printing of paper money gets out of control and we end in a hyper-inflationary fiat money collapse.
Restrictions on gold ownership
Because of its use as a reserve store of value, the possession of gold is sometimes restricted or banned. Within the United States, the private possession of gold except as jewelry and coin collecting was banned between 1933 and 1975. President Franklin D. Roosevelt expropriated gold by Executive Order 6102, and President Richard Nixon closed the gold window by which foreign countries could exchange American dollars for gold at a fixed rate.
Return of a gold standard?
In the first few years of the 21st century, reports started to circulate that Malaysia was planning a return to the gold standard -- to issue and use gold dinars as currency in international trade. The purported purpose of this move would be to reduce dependence on the United States dollar as a reserve currency, and to establish a non-debt-backed currency in accord with Islamic law against the charging of interest. Nonetheless, gold dinar currency has not yet emerged. Privately issued digital gold currency attempt to replicate a gold standard.
Gold in investment portfolios
As a tangible investment gold is sometimes held as part of a portfolio because over the long term gold has an extensive history of maintaining its value. It has in the last century gained ground in relation to fiat currencies owing to inflation. Gold becomes particularly desirable in times of extremely weak confidence and during hyperinflation because gold maintains its value even as fiat money becomes worthless. People who enjoy investing in gold are known as goldbugs.
Futures contracts based on gold currently trade on various exchanges around the world. In the US this occurs primarily on COMEX (Commodity Exchange) which is a subsidiary of the New York Mercantile Exchange. Speculation about the future price of gold and other commodities is carried on at COMEX. Recently, gold-based ETFs like GLD have emerged as a more convenient investment vehicle.
In some countries such as Switzerland, it is possible to hold physical gold as part of an investment portfolio, due to the absence of taxes and narrow bid-ask spreads, however in other countries portfolio managers sometimes hold gold shares or gold bullion securities as a proxy for the metal itself. Exchange Traded Funds such as Gold Bullion Securities are securities sponsored by the World Gold Council and which are fully backed up by allocated gold held by a custodian. The main Gold Bullion Securities are as follows:
Gold nuggets California (top) Australia (bottom) showing octahedral formations
Occurrence
Due to its relative chemical inertness gold is usually found as the native metal or alloy. Occasionally large accumulations of native gold (also known as nuggets) occur but usually gold occurs as minute grains. These grains occur between mineral grain boundries or as inclusions within minerals. Common gold associations are quartz often as veins and sulfide minerals. The most common sulfide associations are pyrite, chalcopyrite, galena, sphalerite, arsenopyrite, stibnite and pyrrhotite. Rarer mineral associations are petzite, calaverite, sylvanite, muthmannite, nagyagite and krennerite.
Gold is widely distributed in the Earth's crust at a background level of 0.03 g/1000 kg (0.03 ppm by weight). Hydrothermal ore deposits of gold occur in metamorphic rocks and igneous rocks; alluvial deposits and placer deposits originate from these sources. The primary source of gold is usually igneous rocks or surface concentrations. A deposit usually needs some form of secondary enrichment to form an economically viable ore deposit: either chemical or physical processes like erosion or solution or more generally metamorphism, which concentrates the gold in sulfide minerals or quartz. There are several primary deposit types, common ones are termed reef or vein. Primary deposits can be weathered and eroded, with most of the gold being transported into stream beds where it congregates with other heavy minerals to form placer deposits. In all these deposits the gold is in its native form. Another important ore type is in sedimentary black shale and limestone deposits containing finely disseminated gold and other platinum group metals.
Gold occurs in sea water at 0.1 to 2 mg/t (0.1 to 2 ppb by weight) depending on sample location.
Production
Economic gold extraction can be achieved from ore grades as little as 0.5 g/1000 kg (0.5 ppm) on average in large easily mined deposits, typical ore grades in open-pit mines are 1–5 g/1000 kg (1-5 ppm), ore grades in underground or hard rock mines are usually at least 3 g/1000 kg (3 ppm) on average. Ore grades of 30 g/1000 kg (30 ppm) are usually needed before gold is visible to the naked eye, therefore in most gold mines you will not see any gold. It is claimed, that all the gold that has been mined throughout the history of mankind could be incorporated in a solid ball with a diameter of 27 metres.
Since the 1880s South Africa has been the source for a large proportion of the world's gold supply. Production in 1970 accounted for 79% of the world supply, producing about 1,000 tonnes, however production in 2004 was 342 tonnes. This decline was due to the increasing difficulty of extraction and changing economic factors affecting the industry in South Africa.
The city of Johannesburg was built atop the world's greatest gold finds. Gold fields in the Orange Free State and the Transvaal are deep and require the world's deepest mines. The Second Boer War of 1899–1901 between the British Empire and the white Boers was at least partly over the rights of miners and possession of the gold wealth in South Africa.
Other major producers are Canada, United States and Western Australia. Mines in South Dakota and Nevada supply two-thirds of gold used in the United States. Siberian regions of the USSR also used to be significant in the global gold mining industry. Kolar Gold Fields in India is another example of a city being built on the greatest gold deposits in India. In South America, the controversial project Pascua Lama aims at exploitation of rich fields in the high mountains of Atacama, at the border between Chile and Argentina.
The idea of producing gold out of lesser metals or other cheap substances has fascinated people throughout the centuries. Scientists, kings and charlatans obsessed with the secret art of alchemy accidentally invented practically useful materials (e.g. porcelain), while searching in vain for the philosopher's stone, which was supposed to turn mercury into gold. Modern science has since proven the impossibility of making gold from other elements via chemical reactions.
However, it is possible to obtain infinitesimally small amounts of gold by artificial nuclear transformations in particle accelerators The gold isotopes produced would likely be radioactive. No economically feasible method to manufacture gold artificially has been found and published yet. The possibility of cheap man-made gold would have unforeseen economic and consequences.
The world's oceans hold a vast amount of gold, but in very low concentrations (perhaps 1-2 parts per billion). Fritz Haber (the German inventor of the Haber process) attempted commercial extraction of gold from sea water in an effort to help pay Germany's reparations following the First World War. Unfortunately, his assessment of the concentration of gold in sea water was unduly high, probably due to sample contamination. The effort produced little gold and cost the German government far more than the commercial value of the gold recovered. No commercially viable mechanism for performing gold extraction from sea water has yet been identified.
Compounds/isotopes
Although gold is a noble metal, it can form many compounds, auric chloride (AuCl3) and chlorauric acid (HAuCl4) being the most common. Gold compounds can be aurous (univalent, +1) or auric (trivalent, +3). Gold also can under extreme conditions form a +5 state with fluorine (gold pentafluoride, AuF5), as well as (unusually for a metal), a -1 state. Such compounds containing the Au- anion are called aurides and include caesium auride, CsAu, rubidium auride, RbAu, and tetramethylammonium auride, (CH3)4N+ Au-. Gold also forms:
There is only one stable isotope of gold, and 18 radioisotopes with Au-195 being the most stable with a half-life of 186 days.
Precautions
The human body doesn't absorb gold very well, thus compounds of gold are not normally very toxic. Liver and kidney damage has, however, been reported for up to 50% of arthritis patients treated with gold-containing drugs. Gold used in dentistry is widely regarded as the safest form of restorative material, as well as the most successful.
Symbolism
Gold has been associated with the extremities of utmost evil and great sanctity throughout history. The Golden Calf is a widely-recognised symbol of idolatry and revolt against God. In Communist propaganda, the golden pocket watch and its fastening golden chain were the characteristic accessories of the class enemy, the bourgeois and the industrial tycoons. American Indians of the Sioux tribe called it "The yellow metal that makes the white man crazy"
On the other hand, eminent orators such as John Chrysostom were said to have a mouth of gold with a silver tongue. Gold is associated with notable anniversaries, particularly in a 50 year cycle, such as a golden wedding anniversary, golden jubilee, etc.
Great human achievements are frequently rewarded with gold, in the form of medals and decorations. Winners of races and prizes are usually awarded the gold medal (such as the Olympic Games and the Nobel Prize), while many award statues are depicted in gold (such as the Academy Awards, the Emmy Awards and the British Academy Film Awards). Medieval kings were inaugurated under the signs of sacred oil and a golden crown, the latter symbolizing the eternal shining light of heaven and thus a Christian king's divinely inspired authority. Wedding rings are traditionally made of gold; since it is long-lasting and unaffected by the passage of time, it is considered a suitable material for everyday wear as well as a metaphor for the relationship. In Orthodox Christianity, the wedded couple is adorned with a golden crown during the ceremony, an amalgamation of symbolic rites.
The symbolic value of gold varies wildly around the world, even within geographic regions. For example, gold is quite common in Turkey but considered a most valuable gift in Sicily.
DIAMOND
Round-brilliant cut diamonds show off reflecting facets
Diamond is one of the two best known forms (or allotropes) of carbon, whose hardness and high dispersion of light make it useful for industrial applications and jewelry (the other equally well known allotrope is graphite). Diamonds are specifically renowned as a mineral with superlative physical qualities - they make excellent abrasives because they can only be scratched by other diamonds, which also means they hold a polish extremely well and retain luster. About 130 million carats (26,000 kg) are mined annually, with a total value of nearly USD $9 billion.
The name "diamond" derives from the ancient Greek adamas (αδάμας; "impossible to tame"). They have been treasured as gems since their use as religious icons in India at least 2,500 years ago—and usage in drill bits and engraving tools also dates to early human history. Popularity of diamonds has risen since the 19th century because of improved cutting and polishing techniques, and they are commonly judged by the "four Cs": carat, clarity, color, and cut. Although nearly four times the mass of natural diamonds are produced as synthetic diamond each year, the vast majority of synthetic diamond production remains small, imperfect diamonds suitable only for industrial-grade use, with gem-quality synthetic diamonds only recently becoming available.
Most natural diamonds originate from central and southern Africa, although significant sources of the mineral have been discovered in Canada, Russia, Brazil, and Australia. They are generally mined from volcanic pipes, which are deep in the Earth where the high pressure and temperature enables the formation of the crystals. The mining and distribution of natural diamonds are subjects of frequent controversy—such as with concerns over the sale of conflict diamonds by African paramilitary groups. There are also allegations that the De Beers Group misuses its dominance in the industry to control supply and manipulate price via monopolistic practices.
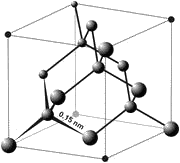
The unit cell of the diamond crystal
Material properties
Diamond is a transparent crystal of pure carbon consisting of tetrahedrally bonded carbon atoms. Humans have been able to adapt diamonds for many uses because of the material's exceptional physical characteristics. Most notable among these properties are the extreme hardness of diamond, its high dispersion index, and high thermal conductivity. These properties form the basis for most modern applications of diamond.
Mechanical properties
Crystal structure
Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Lonsdaleite is a polymorph of diamond (and a distinct mineral species) that crystallizes with hexagonal symmetry; it is rarely found in nature, but is characteristic of synthetic diamonds. A cryptocrystalline variety of diamond is called carbonado. A colorless, grey or black diamond with a tiny radial structure is a spherulite.
The tetrahedral arrangement of atoms in a diamond crystal is the source of many of diamond's properties; graphite, another allotrope of carbon, has a rhombohedral crystal structure and as a result shows dramatically different physical characteristics—contrary to diamond, graphite is a very soft, dark grey, opaque mineral.
Hardness
Diamond is the hardest known naturally occurring material, scoring 10 on the relative Mohs scale of mineral hardness and having an absolute hardness value of between 167 and 231 gigapascals in various tests. Diamond's hardness has been known since antiquity, and is the source of its name. However, aggregated diamond nanorods, an allotrope of carbon first synthesized in 2005, are now believed to be even harder than diamond.
The hardest diamonds in the world are diamonds from the New England area in New South Wales, Australia. These diamonds are generally small, perfect to semiperfect octahedra and are used to polish other diamonds. Their hardness is considered to be a product of the crystal growth form, which is single stage growth crystal. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws and defect planes in the crystal lattice all of which affect their hardness (Taylor et al. 1990).
Industrial use of diamonds has historically been associated with their hardness; this property makes diamond the ideal material for cutting and grinding tools. It is one of the most known and most useful of more than 3,000 known minerals. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial adaptations of this ability include diamond-tipped drill bits and saws, or use of diamond powder as an abrasive. Other specialized applications also exist or are being developed, including use as semiconductors: some blue diamonds are natural semiconductors, in contrast to most other diamonds, which are excellent electrical insulators. Industrial-grade diamonds are either unsuitable for use as gems or synthetically produced, which lowers their price and makes their use economically feasible. Industrial applications, especially as drill bits and engraving tools, also date to ancient times.
The hardness of diamonds also contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well, keeping its luster over long periods of time. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in an engagement ring or wedding ring, which are often worn everyday.
Toughness
Unlike hardness, which only denotes resistance to scratching, diamond's toughness is only fair to good. Toughness relates to a material's ability to resist breakage from forceful impact. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamonds cut into certain particular shapes are therefore more prone to breakage than others.
Color
Diamonds occur in a variety of transparent hues — colorless, white, steel, blue, yellow, orange, red, green, pink, brown—or colored black. Diamonds with a detectable hue to them are known as colored diamonds. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice. The most common impurity, nitrogen, causes a yellowish or brownish tinge.
Thermodynamic stability
At surface air pressure (one atmosphere), diamonds are not as stable as graphite, and so the decay of diamond is thermodynamically favorable (ΔG = −2.99 kJ / mol). Diamonds will burn at approximately 800 degrees Celsius, providing that enough oxygen is available. This was shown in the late 18th century, and previously described during Roman times. So, despite the popular advertising slogan, diamonds are not forever. However, owing to a very large kinetic energy barrier, diamonds are metastable; under normal conditions, it would take an extremely long time (possibly more than the age of the Universe) for diamond to decay into graphite.
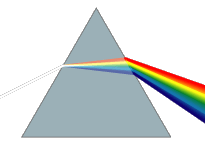
Diamonds exhibit high dispersion of visible light
Optical properties
Diamonds exhibit a high dispersion of visible light. This strong ability to split white light into its component colors is an important aspect of diamond's attraction as a gemstone, giving it impressive prismatic action that results in so-called fire in a well-cut stone. The luster of a diamond, a characterization of how light interacts with the surface of a crystal, is brilliant and is described as adamantine, which simply means diamond-like.
This is owed to their high refractive index of 2.417 (at 589.3 nm), which causes total internal reflection to occur. Some diamonds exhibit fluorescence of various colors (predominately blue) under long wave ultraviolet light. Nearly all diamonds fluoresce bluish-white, yellow or green under X-rays and this property is used extensively in mining to separate the fluorescing diamond from the non-fluorescing rock. Most diamonds show no fluorescence although colored diamonds show a wider range of fluorescence than the blue fluorescence normally observed in clear diamonds
Electrical properties
Except for most blue diamonds, which are semiconductors, diamonds are good electrical insulators. Blue diamonds owe their semiconductive property to boron impurities, which act as a doping agent and cause p-type semiconductor behavior. Blue diamonds which are not boron-doped, such as those recently recovered from the Argyle diamond mine in Australia that owe their color to an overabundance of hydrogen atoms, are not semiconductors.
Thermal properties
Unlike
most electrical insulators, diamond is a good conductor of heat
because of the strong covalent bonding within the crystal. Most
natural blue diamonds contain boron
atoms which replace carbon atoms in the crystal matrix, and also have
high thermal conductivity. Specially purified synthetic diamond has
the highest thermal
conductivity (2000–2500 W/(m·K), five times more than copper)
of any known solid at room temperature. Because diamond has such high
thermal conductance it is already used in semiconductor manufacture to
prevent silicon and other semiconducting materials from overheating.
Natural history
Formation
Diamond is formed by prolonged exposure of carbon bearing materials to high pressure and temperature. On Earth, the formation of diamonds is possible because there are regions deep within the Earth that are at a high enough pressure and temperature that the formation of diamonds is thermodynamically favorable. Under continental crust, diamonds form starting at depths of about 150 kilometers (90 miles), where pressure is roughly 5 gigapascals and the temperature is around 1200 degrees Celsius (2200 degrees Fahrenheit). Diamond formation under oceanic crust takes place at greater depths because of higher temperatures, which require higher pressure for diamond formation. Long periods of exposure to these high pressures and temperatures allow diamond crystals to grow larger.
Through studies of carbon isotope ratios (similar to the methodology used in carbon dating) except using the stable isotopes C-12 and C-13, it has been shown that the carbon found in diamonds comes from both inorganic and organic sources. Some diamonds, known as harzburgitic, are formed from inorganic carbon originally found deep in the Earth's mantle. In contrast, eclogitic diamonds contain organic carbon from organic detritus that has been pushed down from the surface of the Earth's crust through subduction (see plate tectonics) before transforming into diamond. These two different source carbons have measurably different 13C:12C ratios. Diamonds that have come to the Earth's surface are generally very old, ranging from under 1 billion to 3.3 billion years old.
Diamonds occur most often as euhedral or rounded octahedra and twinned octahedra known as macles. As diamond's crystal structure has a cubic arrangement of the atoms, they have many facets that belong to a cube, octahedron, rhombicosidodecahedron, tetrakis hexahedron or disdyakis dodecahedron. The crystals can have rounded off and unexpressive edges and can be elongated. Sometimes they are found grown together or form double "twinned" crystals grown together at the surfaces of the octahedron. This is all due to the conditions in which they form. Diamonds (especially those from secondary deposits) are commonly found coated in nyf, an opaque gum-like skin.
Diamonds can also form in other natural high-pressure, high-temperature events. Very small diamonds, known as microdiamonds or nanodiamonds, have been found in impact craters where meteors strike the Earth and create shock zones of high pressure and temperature where diamond formation can occur. Microdiamonds are now used as one indicator of ancient meteorite impact sites.
Misshapen octahedral rough diamond crystal in matrix
Surfacing
Diamond-bearing rock is forced close to the surface through deep-origin volcanic eruptions. The magma for such a volcano must originate at a depth where diamonds can be formed, 90 miles (150 km) deep or more (three times or more the depth of source magma for most volcanoes); this is a relatively rare occurrence. Below these typically small surface volcanic craters are formations known as volcanic pipes, which contain material that was pushed toward the surface of the earth by volcanic action, but did not erupt before the volcanic activity ceased. Diamond-bearing volcanic pipes are most commonly found in the oldest regions of continental crust, which relates to the fact that these areas are the coolest portions of the earth's crust, and therefore diamonds can form at the shallowest depths.
The magma in such volcanic pipes is usually one of two characteristic types, which cool into igneous rock known as either kimberlite or lamproite. The magma itself does not contain diamond; instead, it acts as an elevator that carries deep-formed rocks and material upward. These rocks are characteristically rich in magnesium bearing olivine, pyroxene, and amphibole minerals which are usually altered to serpentine under near surface conditions. Certain indicator minerals typically occur within diamondiferous kimberlites and are used as mineralogic tracers in the search for diamond deposits by prospectors. These minerals are rich in chromium (Cr) or titanium (Ti), elements which impart bright colors to the minerals. The most common indicator minerals are chromian garnets (usually bright red Cr-pyrope, and occasionally green ugrandite-series garnets), eclogitic garnets, orange Ti-pyrope, red high chromian spinels, dark chromite, bright green Cr-diopside, glassy green olivine, black picroilmenite, and magnetite. Kimberlite deposits are known as blue ground for the deeper serpentinized part of the deposits, or as yellow ground for the near surface smectite clay and carbonate weathered and oxidized portion.
Once diamonds have been forced to the surface by magma in a volcanic pipe, they may erode out and be distributed over a large area. A volcanic pipe containing diamonds is known as a primary source of diamonds. Secondary sources of diamonds include all areas where a significant number of diamonds, eroded out of their kimberlite or lamproite matrix, accumulate because of water or weather action. These include alluvial deposits and deposits along existing and ancient shorelines, where loose diamonds tend to accumulate because of their approximate size and density. Diamonds have also rarely been found in deposits left behind by glaciers (notably in Wisconsin and Indiana); however, in contrast to alluvial deposits, glacial deposits are not known to be of significant concentration and are therefore not viable commercial sources of diamond.
Diamonds can also be brought to the surface through certain processes which may occur when two continental plates collide forcefully, although this phenomenon is less understood and currently assumed to be uncommon.
Gemological characteristics
The use of diamonds as gemstones of decorative value is the most familiar use to most people today, and is also the earliest use, with decorative use of diamonds stretching back into antiquity. The dispersion of white light into a rainbow of colors, known in the trade as fire, is the other primary characteristic of gem diamonds, and has been highly prized throughout history. Over time, especially since around 1900, experts in the field of gemology have developed methods of characterizing diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the four Cs, are now commonly used as the basic descriptors of diamonds: these are carat, clarity, color, and cut.
Most gem diamonds are traded on the wholesale market based on single values for each of the four Cs; for example knowing that a diamond is rated as 1.5 carats, VS2 clarity, F color, excellent cut, is enough to reasonably establish an expected price range. More detailed information from within each characteristic can then be used to determine actual market value for individual stones. Consumers who purchase individual diamonds are often advised to use the four Cs to pick the diamond that is "right" for them; to these is sometimes added the "fifth C" of cost.
Other characteristics not described by the four Cs can and do influence the value or appearance of a gem diamond. These characteristics include physical characteristics such as the presence of fluorescence, as well as data on a diamond's history including its source and which gemological institute performed evaluation services on the diamond. Cleanliness also dramatically affects a diamond's beauty.
There are four major gemological associations which "certify" diamonds: that is, define the four Cs of a diamond. While carat weight and cut angles are mathematically defined, the clarity and color are judged by the trained human eye and are therefore open to slight variance in interpretation.
Carat
The carat weight measures the mass of a diamond. One carat is defined as exactly 200 milligrams (about 0.007 ounce). The point unit—equal to one one-hundredth of a carat (0.01 carat, or 2 mg)—is commonly used for diamonds of less than one carat. All else being equal, the value of a diamond increases exponentially in relation to carat weight, since larger diamonds are both rarer and more desirable for use as gemstones. A review of comparable diamonds available for purchase in September 2005 demonstrates this effect (approximate prices for round cut, G color, VS2 diamonds with "1A" cut grade, as listed on http://www.pricescope.com):
The price per carat does not increase smoothly with increasing size. Instead, there are sharp jumps around milestone carat weights, as demand is much higher for diamonds weighing just more than a milestone than for those weighing just less. As an example, a 0.95 carat diamond may have a significantly lower price per carat than a comparable 1.05 carat diamond, because of differences in demand.
A weekly price list published by Rapaport of New York, of diamond prices per carat, for different diamond cuts, clarity and weights, is currently considered the de-facto retail price baseline. Jewelers often trade diamonds at negotiated discounts off the Rapaport price (e.g., "R -3%").
In the wholesale trade of gem diamonds, carat is often used in denominating lots of diamonds for sale. For example, a buyer may place an order for 100 carats of 0.5 carat, D–F, VS2-SI1, excellent cut diamonds, indicating he wishes to purchase 200 diamonds (100 carats total mass) of those approximate characteristics. Because of this, diamond prices (particularly among wholesalers and other industry professionals) are often quoted per carat, rather than per stone.
Total carat weight (t.c.w.) is a phrase used to describe the total mass of diamonds or other gemstone in a piece of jewelry, when more than one gemstone is used. Diamond solitaire earrings, for example, are usually quoted in t.c.w. when placed for sale, indicating the mass of the diamonds in both earrings and not each individual diamond. T.c.w. is also widely used for diamond necklaces, bracelets and other similar jewelry pieces.
Clarity
Clarity is a measure of internal defects of a diamond called inclusions. Inclusions may be crystals of a foreign material or another diamond crystal, or structural imperfections such as tiny cracks that can appear whitish or cloudy. The number, size, color, relative location, orientation, and visibility of inclusions can all affect the relative clarity of a diamond. The Gemological Institute of America (GIA) and others have developed systems to grade clarity, which are generally based on those inclusions which are visible to a trained professional when a diamond is viewed from above, under 10x magnification.
Diamonds become increasingly rare when considering higher clarity gradings. Only about 20 percent of all diamonds mined have a clarity rating high enough for the diamond to be considered appropriate for use as a gemstone; the other 80 percent are relegated to industrial use. Of that top 20 percent, a significant portion contains an inclusion or inclusions that are visible to the naked eye upon close inspection. Those that do not have a visible inclusion are known as "eye-clean" and are preferred by most buyers, although visible inclusions can sometimes be hidden under the setting in a piece of jewelry.
Most inclusions present in gem-quality diamonds do not affect the diamonds' performance or structural integrity. However, large clouds can affect a diamond's ability to transmit and scatter light. Large cracks close to or breaking the surface may reduce a diamond's resistance to fracture. Diamonds are graded by the major societies on a scale ranging from Flawless to Imperfect.
The Hope Diamond
Color
A chemically pure and structurally perfect diamond is perfectly transparent with no hue, or color. However, in reality almost no gem-sized natural diamonds are absolutely perfect. The color of a diamond may be affected by chemical impurities and/or structural defects in the crystal lattice. Depending on the hue and intensity of a diamond's coloration, a diamond's color can either detract from or enhance its value. For example, most white diamonds are discounted in price as more yellow hue is detectable, while intense pink or blue diamonds (such as the Hope Diamond) can be dramatically more valuable.
Most diamonds used as gemstones are basically transparent with little tint, or white diamonds. The most common impurity, nitrogen, replaces a small proportion of carbon atoms in a diamond's structure and causes a yellowish to brownish tint. This effect is present in almost all white diamonds; in only the rarest diamonds is the coloration due to this effect undetectable. The GIA has developed a rating system for color in white diamonds, from "D" to "Z" (with D being "colorless" and Z having a bright yellow coloration), which has been widely adopted in the industry and is universally recognized, superseding several older systems once used in different countries.
The system uses a benchmark set of either natural diamonds of known color grade, or precision-crafted cubic zirconia; test lighting conditions are also standardized and carefully controlled. Diamonds with higher color grades are rarer, in higher demand, and therefore more expensive, than lower color grades. Oddly enough, diamonds graded Z are also rare, and the bright yellow color is also highly valued. Diamonds graded D-F are considered "colorless", G-J are considered "near-colorless", K-M are "slightly colored". N-Y are usually appear light yellow or brown.
In contrast to yellow or brown hues, diamonds of other colors are much rarer and more valuable. While even a pale pink or blue hue may increase the value of a diamond, more intense coloration is usually considered more desirable and commands the highest prices. A variety of impurities and structural imperfections cause different colors in diamonds, including yellow, pink, blue, red, green, brown, and other hues. Diamonds with unusual or intense coloration are sometimes labeled "fancy" by the diamond industry. Intense yellow coloration is considered one of the fancy colors, and is separate from the color grades of white diamonds. Gemologists have developed rating systems for fancy colored diamonds, but they are not in common use because of the relative rarity of colored diamonds.
Cut
Diamond cutting is the art and science of creating a gem-quality diamond out of mined rough. The cut of a diamond describes the manner in which a diamond has been shaped and polished from its beginning form as a rough stone to its final gem proportions. The cut of a diamond describes the quality of workmanship and the angles to which a diamond is cut. Often diamond cut is confused with "shape."
There are mathematical guidelines for the angles and length ratios at which the diamond is supposed to cut at in order to reflect the maximum amount of light. Round brilliant diamonds, the most common, are guided by these specific guidelines, though fancy cut stones are not able to be as accurately guided by mathematical specifics.
The techniques for cutting diamonds have been developed over hundreds of years, with perhaps the greatest achievements made in 1919 by mathematician and gem enthusiast Marcel Tolkowsky. He developed the round brilliant cut by calculating the ideal shape to return and scatter light when a diamond is viewed from above. The modern round brilliant has 57 facets (polished faces), counting 33 on the crown (the top half), and 24 on the pavilion (the lower half). The girdle is the thin unpolished middle. The function of the crown is to diffuse light into various colors and the pavilion's function to reflect light back through the top of the diamond.
Tolkowsky defines the ideal dimensions to have:
The culet is the tiny point at the bottom of the diamond. This should be a negligible diameter, otherwise light leaks out of the bottom. Tolkowsky's ideal dimensions did not include a girdle. However, a thin girdle is required in reality in order to prevent the diamond from easily chipping in the setting. A normal girdle should be about 1%–2% of the overall diameter.
The further the diamond's characteristics are from Tolkowsky's ideal, the less light will be reflected. However, there is a small range in which the diamond can be considered "ideal." Today, because of the relative importance of carat weight in society, many diamonds are often intentionally cut poorly to increase carat weight. There is a financial premium for a diamond that weighs the magical 1.0 carat, so often the girdle is made thicker or the depth is increased. Neither of the these tactics make the diamond appear any bigger, but it also greatly reduces the sparkle of the diamond. So a poorly cut 1.0 carat diamond may have the same diameter and appear as large as a 0.85 carat diamond. The depth percentage is the overall quickest indication of the quality of the cut of a round brilliant. "Ideal" round brilliant diamonds should not have a depth percentage greater than 62.5%. Another quick indication is the overall diameter.
Typically a round brilliant 1.0 carat diamond should have a diameter of about 6.5 mm. Mathematically, the diameter in millimeters of a round brilliant should approximately equal 6.5 times the cube root of carat weight, or 11.1 times the cube root of gram weight.
Shape
Diamonds do not show all of their beauty as rough stones; instead, they must be cut and polished to exhibit the characteristic fire and brilliance that diamond gemstones are known for. Diamonds are cut into a variety of shapes that are generally designed to accentuate these features.
Diamonds which are not cut to the specifications of Tolkowsky's round brilliant shape (or subsequent variations) are known as "fancy cuts." Popular fancy cuts include the baguette (from the French, resembling a loaf of bread), marquise, princess (square outline), heart, briolette (a form of the rose cut), and pear cuts. Generally speaking, these "fancy cuts" are not held to the same strict standards as Tolkowsky-derived round brilliants and there are less specific mathematical guidelines of angles which determine a well-cut stone.
Cuts are influenced heavily by fashion: the baguette cut—which accentuates a diamond's luster and downplays its fire—was all the rage during the Art Deco period, whereas the princess cut—which accentuates a diamond's fire rather than its luster—is currently gaining popularity. The princess cut is also popular amongst diamond cutters: of all the cuts, it wastes the least of the original crystal. The past decades have seen the development of new diamond cuts, often based on a modification of an existing cut. Some of these include extra facets. These newly developed cuts are viewed by many as more of an attempt at brand differentiation by diamond sellers, than actual improvements to the state of the art.
Quality
The quality of a diamond's cut is widely considered the most important of the four Cs in determining the beauty of a diamond; indeed, it is commonly acknowledged that a well-cut diamond can appear to be of greater carat weight, and have clarity and color appear to be of better grade than they actually are. The skill with which a diamond is cut determines its ability to reflect and refract light.
In addition to carrying the most importance to a diamond's quality as a gemstone, the cut is also the most difficult to quantitatively judge. A number of factors, including proportion, symmetry, and the relative angles of various facets, are determined by the quality of the cut and can affect the performance of a diamond. A poorly cut diamond with facets cut only a few degrees out of alignment can result in a poorly performing stone. For a round brilliant cut, there is a balance between "brilliance" and "fire." When a diamond is cut for too much "fire," it looks like a cubic zirconia, which gives off much more "fire" than real diamond. A well executed round brilliant cut should reflect most light out from the tabletop and make the diamond appear white when viewed from the top. An inferior cut will produce a stone that appears dark at the center and in some extreme cases the ring settings may show through the top of the diamond as shadows.
Several different theories on the "ideal" proportions of a diamond have been and continue to be advocated by professional gemologists. Recently, there has been a shift away from grading cut by the use of various angles and proportions toward measuring the performance of a cut stone. A number of specially modified viewers have been developed toward this end. One result of this trend is the rise of the phrase "hearts and arrows," describing a characteristic pattern observable on stones exhibiting high symmetry. Hearts and arrows diamonds trade at a 10 to 20 percent premium to otherwise comparable diamonds.
An uncut diamond does not show its prized optical properties
The cutting process
The process of shaping a rough diamond into a polished gemstone is both an art and a science. The choice of cut is often decided by the original shape of the rough stone, location of the inclusions and flaws to be eliminated, the preservation of the weight, popularity of certain shapes amongst consumers and many other considerations. The round brilliant cut is preferred when the crystal is an octahedron, as often two stones may be cut from one such crystal. Oddly shaped crystals such as macles are more likely to be cut in a fancy cut, that is, a cut other than the round brilliant, which the particular crystal shape lends itself to.
Even with modern techniques, the cutting and polishing of a diamond crystal always results in a dramatic loss of weight; rarely is it less than 50%. Sometimes the cutters compromise and accept lesser proportions and symmetry in order to avoid inclusions or to preserve the carat rating. Since the per carat price of diamond shifts around key milestones (such as 1.00 carat), many one-carat diamonds are the result of compromising "Cut" for "Carat." Some jewelry experts advise consumers to buy a 0.99 carat diamond for its better price or buy a 1.10 carat diamond for its better cut, avoiding a 1.00 carat diamond which is more likely to be a poorly cut stone.
Cleaning
Although it is not one of the four Cs, cleanliness affects a diamond's beauty as much as any of the four Cs. A clean diamond is more brilliant and fiery than the same diamond when it is "dirty". Dirt or grease on the top of a diamond reduces its luster. Water, dirt, or grease on the bottom of a diamond interferes with the diamond's brilliance and fire. Even a thin film absorbs some light that could have been reflected to the person looking at the diamond. Colored dye or smudges can affect the perceived color of a diamond. Historically, some jewelers' stones were misgraded because of smudges on the girdle, or dye on the culet. Current practice is to thoroughly clean a diamond before grading its color.
Maintaining a clean diamond can sometimes be difficult, as jewelry settings can obstruct cleaning efforts, and oils, grease, and other hydrophobic materials adhere well to a diamond's surface. Some jewelers provide their customers with ammonia-based cleaning kits; ultrasonic cleaners are also popular.
Cleanliness does not affect the diamond's market value, as any competent jeweler will clean the diamond before offering it for sale. However, cleanliness might reflect a diamond's sentimental value: some jewelers have noted a correlation between ring cleanliness and marriage quality.
History
Diamonds are thought to have been first recognized and mined in India, where significant alluvial deposits of the stone could then be found. The earliest written reference can be found in the text Arthasastra, which was completed around 296 BCE, describes diamond's hardness, luster, and dispersion. Diamonds quickly became associated with divinity, being used to decorate religious icons, and were believed to bring good fortune to those who carried them. Ownership was restricted among various castes by color, with only kings being allowed to own all colors of diamond.
In February 2005, a joint Chinese-U.S. team of archaeologists reported the discovery of four corundum-rich stone ceremonial burial axes originating from China's Liangzhu and Sanxingcun cultures (4000 BCE–2500 BCE) which, because of the axes' specular surfaces, the scientists believe were polished using diamond powder. Although there are diamond deposits now known to exist close to the burial sites, no direct evidence of coeval diamond mining has been found: the researchers came to this conclusion by polishing corundum using various lapidary abrasives and modern techniques then comparing the results using an atomic force microscope. At that scale, the surface of the modern diamond-polished corundum closely resembled that of the axes; however, the polishes of the latter were superior.
Diamonds were traded to both the east and west of India and were recognized by various cultures for their gemological or industrial uses. The Roman writer Pliny the Elder noted diamond's ornamental uses, as well as its usefulness to engravers because of its hardness, in his work Naturalis Historia. In China, diamonds seem to have been used primarily for engraving jade and drilling holes in beads. Archeological evidence from Yemen suggests that diamonds were used as drill tips as early as the 4th century BCE. In Europe, however, diamonds disappeared for almost 1,000 years following the rise of Christianity because of two effects: early Christians rejected diamonds because of their earlier use in amulets, and Arabic traders restricted the flow of trade between Europe and India.
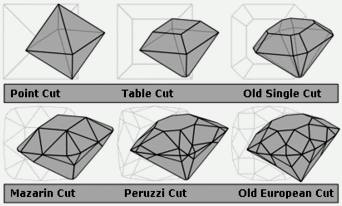
Diamond cuts showing the evolution from the most primitive (point cut) to the most advanced pre-Tolkowsky cut (old European)
Until the late Middle Ages, diamonds were most prized in their natural octahedral state, perhaps with the crystal surfaces polished to increase luster and remove foreign material. Around 1300, the flow of diamonds into Europe increased via Venice's trade network, with most flowing through the low country ports of Bruges, Antwerp, and Amsterdam. During this time, the taboo against cutting diamonds into gem shapes, which was established over 1,000 years earlier in the traditions of India, ended allowing the development of diamond cutting technology to begin in earnest.
By 1375, a guild of diamond polishers had been established at Nuremberg. Over the following centuries, various diamond cuts were introduced which increasingly demonstrated the fire and brilliance that makes diamonds treasured today: the table cut, the briolette (around 1476), the rose cut (mid-16th century), and by the mid-17th century, the Mazarin, the first brilliant cut diamond design. In 1919, Marcel Tolkowsky developed an ideal round brilliant cut design that has set the standard for comparison of modern gems; however, diamond cuts have continued to be refined.
The rise in popularity of diamonds as gems seems to have paralleled increasing availability through European history. In the 13th century, King Louis IX of France established a law that only the king could own diamonds. However, within a century diamonds were popular gems among the moneyed aristocratic and merchant classes, and by at latest 1477 had begun to be used in wedding rings. Popularity continued to rise as new cuts were developed that enhanced the diamond's aesthetic appeal, and has largely continued unabated to this day; diamonds have proven popular with all classes in society as their cost has become within reach. A number of large diamonds have become historically significant objects, as their inclusion in various sets of crown jewels and the purchase, sale, and sometimes theft of notable diamonds, have sometimes become politicized.
Record-holding diamonds
The Cullinan Diamond, owned by Queen Elizabeth II was the largest gem-quality rough diamond ever found (1905), at 3,106.75 carats. One of the diamonds cut from it, Cullinan I or the Great Star of Africa, was formerly the largest cut diamond at 530.2 carats, but now that title has been taken by the Golden Jubilee (1985), a 545.67 carat yellow-brown diamond. The largest flawless and colorless (grade D) diamond is the Millennium Star (1990) at 203.04 carats.
The diamond industry
The diamond industry can be broadly separated into two basically distinct categories: one dealing with gem-grade diamonds and another for industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets act in dramatically different ways.
Gem diamond industry
A large trade in gem-grade diamonds exists. Unlike precious metals such as gold or platinum, gem diamonds do not trade as a commodity: there is a substantial mark-up in the sale of diamonds, and there is not a very active market for resale of diamonds. One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to a few locations (most importantly New York, Antwerp, London, Tel Aviv, Amsterdam and increasingly Gujarat), and a single company—De Beers—controls over half of all trade in diamonds. They are based in Johannesburg, South Africa and London, England.
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers (the most important being Antwerp). The De Beers company holds a clearly dominant position in the industry, and has done so since soon after its founding in 1888. De Beers owns or controls a significant portion of the world's rough diamond production facilities (mines) and distribution channels for gem-quality diamonds. The company and its subsidiaries own mines that produce some 40 percent of annual world diamond production, and control distribution channels handling nearly two thirds of all gem diamonds. At one time it was thought over 80 percent of the world's rough diamonds passed through the Diamond Trading Company (DTC, a subsidiary of De Beers) in London, but presently the figure is estimated at around 60 percent. De Beers has used its monopoly position to establish strict price controls, and aggressively market diamonds directly to consumers in world markets.
The De Beers diamond advertising campaign is acknowledged as one of the most successful and innovative ones in history. N.W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and opened up new markets, even in countries where no diamond tradition had existed before. N.W. Ayer's multifaceted marketing campaign included product placement, advertising the diamond itself rather than the De Beers brand, and building associations with celebrities and royalty. This coordinated campaign has lasted decades and continues today; it is perhaps best captured by the now-familiar slogan "a diamond is forever".
Industrial diamond industry
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20,000 kg annually), unsuitable for use as gemstones and known as bort, are destined for industrial use. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; another 400 million carats (80,000 kg) of synthetic diamonds are produced annually for industrial use—nearly four times the mass of natural diamonds mined over the same period.
The dominant industrial use of diamond is in cutting, drilling, grinding, and polishing. Most uses of diamonds in these technologies do not require large diamonds; in fact, most diamonds that are gem-quality except for their small size, can find an industrial use. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications. Specialized applications include use in laboratories as containment for high pressure experiments, high-performance bearings, and limited use in specialized windows.
With the continuing advances being made in the production of synthetic diamond, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a semiconductor suitable to build microchips from, or the use of diamond as a heat sink in electronics. Significant research efforts in Japan, Europe, and the United States are under way to capitalize on the potential offered by diamond's unique material properties, combined with increased quality and quantity of supply starting to become available from synthetic diamond manufacturers.
British Imperial Crown (Crown Jewels)
Diamond supply chain
The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world. In fact, the amount of power which De Beers has consolidated historically prevented it from direct trade with the United States, as its trade practices led to an indictment for violating antitrust regulations (the case was settled in 2004). The concentration of power only loosens at the retail level, where diamonds are sold by a limited number of distributors, known as sight-holders, to jewelers around the world. Sources
Historically diamonds were known to be found only in alluvial deposits in southern India; India led the world in diamond production from the time of their discovery in approximately the 9th century BCE to the mid-18th century CE, but the commercial potential of these sources has been exhausted. The first non-Indian diamond source was found in Brazil in 1725. Today, most commercially viable diamond deposits are in Africa, notably in South Africa, Namibia, Botswana, the Republic of the Congo, Angola and Sierra Leone. There are also commercial deposits being actively mined in the Northwest Territories of Canada, Siberia (mostly in Yakutia territory, for example Mir pipe and Udachnaya pipe), Brazil, and in Northern and Western Australia. Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as conflict diamonds or blood diamonds. In response to public concerns that their diamond purchases were contributing to war and human rights abuses in central Africa and west Africa, the diamond industry and diamond-trading nations introduced the Kimberley Process in 2002, which is aimed at ensuring that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups.
The Kimberley Process provides documentation and certification of diamond exports from producing countries to ensure that the proceeds of sale are not being used to fund criminal or revolutionary activities. Although the Kimberly Process has been somewhat successful in limiting the number of conflict diamonds entering the market, conflict diamonds smuggled to market continue to persist to some degree.
Currently, gem production totals nearly 30 million carats (6,000 kg) of cut and polished stones annually, and over 100 million carats (20,000 kg) of diamonds are sold for industrial use each year. In 2003, this constituted total production of nearly US$9 billion in value.
Distribution
The Diamond Trading Company, or DTC, is a subsidiary of De Beers and markets rough diamonds produced both by De Beers mines and other mines from which it purchases rough diamond production; in whole, about two thirds of all rough diamonds pass through the company. DTC performs sophisticated sorting of rough diamonds into over 16,000 categories, and then sells bulk lots of rough diamonds to a limited number of sight-holders a few times a year.
Once purchased by sigh-tholders, diamonds are cut and polished in preparation for sale as gemstones. The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, and Thailand. Cutting centers with lower costs of labor, notably Surat in Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. Demonstrating this, India produces 90% of all cut and polished diamonds by number, but only 55% by value. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems than was previously economically feasible.
Diamonds which have been prepared as gemstones are sold on diamond exchanges called bourses. There are 24 registered diamond bourses. This is the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or as is increasingly popular, sold unset ("loose"). According to the Rio Tinto Group, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and retail sales of US$57 billion.
Synthetics, simulants, and enhancements
The gemological and industrial uses of diamond have created a large demand for raw stones. A portion of this demand is now being met by synthetic diamonds, man-made diamonds which have similar properties to natural diamonds. This process has historically produced industrial-grade diamonds, but synthetic diamond producers have recently begun to penetrate the gem diamond market. Diamonds have been manufactured synthetically for over fifty years.
A diamond's gem quality, which is not as dependent on material properties as industrial applications, has invited both imitation and the invention of procedures to enhance the gemological properties of natural diamonds. Materials which have similar gemological characteristics to diamond but are not real mined or synthetic diamond are known as diamond simulants. The most familiar diamond simulant to most consumers is cubic zirconia (commonly abbreviated as CZ); recently moissanite has also gained cachet as a popular diamond simulant. Both CZ and moissanite are synthetically produced for use as a diamond simulant. Diamond enhancements are specific treatments, performed on natural diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.
Currently, trained gemologists with appropriate equipment are able to distinguish natural diamonds from all synthetic and simulant diamonds, and identify all enhanced natural diamonds. The established natural diamond industry has a vested interest in maintaining the distinction between natural diamonds and other diamonds, and has made significant investments toward that end. However, synthetic diamonds may one day be indistinguishable from natural diamonds, and new techniques for simulants (such as coating them with a very thin diamond-like layer of carbon) are making it harder to easily distinguish between simulants and real diamonds.
Symbolism
Because of their extraordinary physical properties, diamonds have been used symbolically since near the time of their first discovery. Perhaps the earliest symbolic use of diamonds was as the eyes of Hindu devotional statues. The diamonds themselves were thought to be endowments from the gods and were therefore cherished. The point at which diamonds began to be associated with divinity is not known, but early texts indicate that it was recognized in India since at least 400 BCE. It is said the Greeks believed diamonds were tears of the gods; the Romans believed they were splinters of fallen stars. Many long dead cultures have sought to explain diamond's superlative properties through divine or mystical affiliations.
In Tibetan Buddhism, also known as Vajrayana (Diamond Vehicle), diamonds are an important symbol, and the Diamond Sutra is one of the most popular texts.
In Western culture, diamonds are the traditional emblem of fearlessness and virtue, but have also often associated with power, wealth, crime and misfortune. Today, diamonds are used to symbolize eternity and love, being often seen adorning engagement rings and sometimes wedding rings as well. The popularity of this modern tradition can be traced directly to the marketing campaigns of De Beers, starting in 1938. The diamond engagement ring is, however, not an original invention of De Beers. It can be traced to the marriage of Maximilian I (then Archduke of Austria) to Mary of Burgundy in 1477.
Other early examples of betrothal jewels incorporating diamonds include the Bridal Crown of Blanche (ca. 1370–80) and the Heftlein brooch of Vienna (ca. 1430–40), a pictorial piece depicting a wedding couple. Inaccessibility of diamonds to the vast majority of the population limited the popularity of diamonds as betrothal jewels during this period.
Diamonds were also a symbol of gay community in the 1950s. The Mattachine Society, one of the first and the foremost gay rights groups in the United States, used so-called harlequin diamonds (four smaller diamonds arranged in a pattern to form one larger diamond) as their emblem.
The LifeGem company further taps modern symbolism by offering to synthetically convert the carbonized remains of people or pets into "memorial diamonds". However, many people feel very uncomfortable at the thought of wearing the carbonized remains of people as jewelry.
The diamond is the birthstone for people born in the month of April, and is also used as the symbol of a sixty-year anniversary, such as a Diamond Jubilee (see hierarchy of precious substances).
Diamonds are a common focus of fiction. Notable pieces of fiction include Ian Fleming's Diamonds Are Forever (1956), Arthur C. Clarke's 2061: Odyssey Three (1988) and Neal Stephenson's The Diamond Age (1995). In addition, diamonds are the subject of various myths and legends.
LINKS
REFERENCE
WEDDING RINGS
A wedding band, or wedding ring consists of a precious metal ring, usually worn on the base of the ring finger -- the fourth finger (with the thumb counted as the first finger). Such a ring symbolises marriage: a spouse wears it to indicate a marital commitment to fidelity. The European custom of wearing such a ring has spread widely beyond Europe. Traditional customsPre-wedding customs
According to some customs, the wedding ring forms the last in a series of gifts which also may include the engagement ring, traditionally given as a betrothal present, and the promise ring, often given when serious courting begins. (Other (more recent) traditions (and the jewelry trade) seek to expand the idea of a series of ring-gifts with an eternity ring, which symbolises the renewal or ongoing nature of a lasting marriage, sometimes given after the birth of a first child, and a trilogy ring (usually) dsplaying three brilliant cut round diamonds each, in turn, representing the past, present and future of a relationship.)
An European tradition encourages the engraving of the name of one's intended spouse and the date of one's intended marriage on the inside surface of wedding rings, thus strengthening the symbolism and sentimentality of the rings as they become family heirlooms.
Wedding ceremony customs
The best man has a traditional duty to keep track of a marrying couple's wedding ring(s) and to produce them at the symbolic moment of the giving and receiving of the ring(s) during the traditional marriage ceremony.
In more grandiose weddings, a ring bearer (usually a young boy) may assist in the ceremonial of parading the ring(s) into the ceremony, often on a special cushion or pillow(s).
Post-wedding customs
Before medical science discovered how the circulatory system functioned, people believed that a vein of blood ran directly from the fourth finger on the left hand to the heart. (This belief allegedly dates to the 3rd century BC in Greece.) Because of the hand-heart connection, people named the putative vein descriptively vena amori, Latin for "the vein of love". Due to this tradition, it became accepted to wear the wedding ring on this finger. By wearing rings on the fourth finger of their left hands, a married couple symbolically declares their eternal love for each other. This has now become a matter of tradition and etiquette.
In most Western cultures, the wedding ring is worn on the left hand. In some countries, however (such as Germany, Norway, and Chile), it is worn on the right hand. Orthodox Christians, East Europeans and Jews also wear the wedding band on the right hand traditionally.
Etiquette frowns severely on the making of sexual overtures to a man or woman wearing a wedding ring.
Contemporary usage
In the United Kingdom and the United States in past generations women wore wedding bands much more commonly than men did. Today, both partners often wear wedding rings, but where occupations or professions forbid or discourage the wearing of jewellery (as in the cases of actors, police and electrical workers) either marriage partner may not wear a ring. In addition, people often remove wedding rings for comfort or safety. So it commonly occurs for chaste married people not to wear a wedding ring. Either partner may wear a wedding ring on a chain around the neck, thus conveying the socially equivalent message to wearing it on a finger.
One interpretation states that the woman wears the wedding ring below the engagement ring, thus making it closer to the heart. Purists hold this practice, though common, as incorrect: they claim no ring should fit above the wedding ring, which should be worn alone.
Materials
Most religious marital ceremonies accept a band of any material (even a rubber band) to symbolise the taking of marriage vows, with unusual substitutions permitted in marriages under unusual circumstances. When people marry on shipboard and cannot obtain or adjust a metal ring of appropriate size, the partners often use rubber bands.
To make wedding rings jewellers most commonly use a precious yellow alloy of gold, hardened with copper, tin and bismuth. Platinum and white alloys of gold class as equivalent or superior to gold. Titanium has recently become a popular material for wedding bands, due to its durability, affordability, and gunmetal grey color. The least expensive material in common use is nickel silver for those who prefer its appearance or cost. Silver, copper, brass and other corroding metals do not occur as frequently because they stain the skin. Marrying couples seldom use stainless steel (which does not count as a precious metal). Aluminum or poisonous metals are almost never used. Rings made by either spouse rank highest; and as a result become so precious to the couple that any material becomes acceptable, even if practically unwearable.
Styles, patterns, fashions
The plain gold band is the most popular pattern. Medical personnel commonly wear it because it can be kept very clean. Woman usually wear narrow bands, while men wear broader bands.
In France and French-speaking countries, a common pattern consists of three interleaved rings. They stand for "faith, hope and love", where love equates to that particular type of perfect love indicated by the ancient Greek word agape. Provocatively, this pattern slides off quickly, because the rings flow over each other. A traditional Irish wedding ring, the Claddagh ring, has become popular in the United States and Australia as well, thanks to Irish immigration to those countries.
Men in Greek, Italian and Anatolian cultures sometimes receive and wear puzzle rings -- sets of interlocking metal bands that one must arrange just so in order to form a single ring. Women wryly give them as a test for their mens' chastity. Even when the man masters the puzzle, he still cannot remove and replace the ring quickly! In North America, many married women wear two rings on the same finger: an engagement ring and a plain wedding band. Couples often purchase such rings as a pair of bands designed to fit together.
Quote
"With this ring I thee wed." -- from the traditional Church of England marriage-ceremony formula.
JEWELLERS ONLINE
Argos Catalogue - Nationwide retailer offering over 9000 quality, branded products to choose from, including furniture, electricals, toys, homewares, jewellery, watches, leisure and much more.
Diamond Daisy - Diamond Daisy sells a stunning range of beautiful modern jewellery in sterling silver and 9ct gold, including designer collections and best-selling brand names. They also offer customers free UK shipping, secure shopping and 30-day no quibble returns.
Diamond rings UK London - Prestigious diamond jewellers. Shop online or visit our London showroom
Dot Jewellery Gifts - Fantastic choice of jewellery gifts to suit all occasions
Find Jewellery - Offering a very nice range of gold and silver earrings, pendants, necklaces, bracelets, birthstones and more. Well worth a visit as prices are often well below those on the high street.
Gems TV - Buy direct from the Manufacturer Beautiful pieces unbeatable prices
Gemstones Factory - Log on and get free estimates of the amount they could pay you for your gems or jewellery. Worldwide offices, to ensure economic postage of your gems and jewelry.
Gerald Online Jewellery - selling high quality diamond jewellery, branded watches and hall-marked gold and silver at exceptionally low prices
Goldsmiths UK Jewellers - Goldsmiths is the largest high quality jewellery retailer in the UK. The company has been trading since 1778 already has over 150 showrooms throughout the UK. They have a huge range of classic and contemporary designs of jewellery as well as an excellent range of leading brand name watches available to view and purchase online.
Great Universal Essentials - At Great Universal you can find 1000s of top brand products in a variety of categories including clothing, footwear and accessories, childrenswear, sportswear, products for the home and garden, home entertainment, toys, games, gifts and jewellery.
Gregory & Co - Mens Cufflinks by Gregory & Co. jewellery rings pendant gold gifts pearls platinum silver necklace bangle.
Harrods Online Sale - Up to 50% off Jewellery - Earrings, Necklaces, Bracelets and more
Jewellery.TV - Jewellery.tv have possibly the largest selection of gold, silver, platinum and diamond jewellery online in the UK. Their range caters for all tastes and budgets with nearly 5000 products covering anklets, bangles, bracelets, brooches, chains, cufflinks, earrings, lockets, navel bars, necklaces, pendants and rings. Competitively priced and offering savings on RRP of up to 70% on jewellery from as little as £3.00 to nearly £5000!
Jovavie Fashion Jewellery - Designer fashion jewellery from Mikey and Dyrberg Kern
Lauren Rose Jewellery - Jewellery at Lauren Rose Jewellery. Discover quality gold jewellery and silver jewellery including rings, earrings, bracelets, bangles, necklaces and pendants with fine gemstones including diamonds, amethysts, garnets and blue topaz.
Links of London - Links of London has established a reputation for innovative and affordable luxury gifts and jewellery. Links of London product range includes men’s and women’s jewellery, watches, accessories and gifts in sterling silver and 18 carat gold. Many of their jewellery pieces feature diamonds, semi-precious stones and unique design features. Links of London gifts feature other materials such and leather or enamel, and range from a flash data disk to a limited edition silver and mahogany Noah’s Ark. There is something for everyone adults and kids alike, fun and serious.
LX Direct - Part of the Littlewoods Shop Direct Group. Offering over 40,000 products including top brand fashions, footwear, electrical appliances, home entertainment, gaming, sportswear & equipment, toys, gifts, jewellery, interiors and garden products.
MadlyDeeply - MadlyDeeply are stockists of the exceptionally popular, fast selling Hot Diamonds (Jewellery of the Year award) as well as specialists in wedding rings, engagement rings, eternity rings, earrings and pendants. A stunning range to please every bride and bride to be is available to purchase online!
PARISMA - High Fashion International Jewellery Collections and Designs, Swarovski Crystal, Contemporary Silver, Fashionably Mediterranean and Glamorous, Handcrafted Designer Jewellery, Bella Tioro Handbags and Bellisima Evening Bags
Silver by Mail UK - Choose and buy from this online jewellery catalogue offering the latest exclusive collection of designer Sterling Silver jewellery from around the world.
Swarovski Online Shop - All of the new products from Swarovski Giftware (Silver Crystal, Crystal Moments and Crystal Paradise), Crystal Decor and Daniel Swarovski Home Accessories, Swarovski Jewelry and Accessories are all available from the Swarovski online shop.
The Beautiful Company - A solely internet based Jewellery retailer with over 2000 products available including diamond rings, engagement and eternity rings, wedding rings, titanium rings and covering bothmens and womens jewellery needs. There is a moneyback guarantee with every purchase and an interest free installment payment option is available as well as payment by credit card, paypal and cheque. Delivery within the UK is also Free
The Diamond Jeweller - The Diamond Jeweller is the one-stop shop for all your jewellery requirements. They have over 650 fantastic items of diamond jewellery in stock including diamond engagement rings, eternity rings, earrings, pendants, necklaces, bracelets and watches. The prices are on average 30% less than the high street, providing fantastic value. All the diamonds they use in their jewellery are "conflict-free" so you can rest assured that they have come from legitimate sources and are the genuine article.
LIST OF DIAMOND MINES
Africa
Asia
North America
Oceania
LIST OF FAMOUS DIAMONDS
A number of large or extraordinarily colored diamonds have gained fame, both as exquisite examples of the beautiful nature of diamonds, and because of the famous people who wore, bought, and sold them. A partial list of famous diamonds in history follows:
A heartwarming action adventure: Pirate whalers V Conservationists, introducing John Storm and his solar powered robot ship as they fight to save a wounded whale from the sushi bars. For release as an e-book from 2013/4 with hopes for a film in 2015 TBA
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
This website is Copyright © 1999 & 2013 Max Energy Limited - an educational charity working hard to protect the planet. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
AUTOMOTIVE | BLUEPLANET | ELECTRIC CARS | ELECTRIC CYCLES | SOLAR CARS | SOLARNAVIGATOR |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||